Systemic disorders in the body. What are autoimmune diseases? List of pathologies. Other diseases from the group Diseases of the musculoskeletal system and connective tissue
Nowadays, a common reason for visiting a doctor is joint pain - rheumatism, Reiter's syndrome, arthritis. There are many reasons for the increase in morbidity, including environmental violations, irrational therapy, and late diagnosis. Systemic connective tissue diseases, or diffuse connective tissue diseases, are a group of diseases characterized by a systemic type of inflammation of various organs and systems, combined with the development of autoimmune and immune complex processes, as well as excessive fibrosis formation.
Group systemic diseases connective tissue includes:
| - systemic lupus erythematosus; |
| - systemic scleroderma; |
| - diffuse fasciitis; |
| - dermatomyositis (polymyositis) idiopathic; |
| - Sjögren's disease (syndrome); |
| - mixed connective tissue disease (Sharpe's syndrome); |
| - polymyalgia rheumatica; |
| - relapsing polychondritis; |
| - recurrent panniculitis (Weber-Christian disease); |
| - Behçet's disease; |
| - primary antiphospholipid syndrome; |
| - systemic vasculitis; |
| - rheumatoid arthritis. |
Modern rheumatology names the following causes of diseases: genetic, hormonal, environmental, viral and bacterial. For successful and effective therapy it is necessary to make a correct diagnosis. To do this, you should contact a rheumatologist, and the sooner the better. Today, doctors are armed with an effective SOIS-ELISA test system, which allows them to carry out high-quality diagnostics. Since very often the cause of joint pain is infectious process caused by various microorganisms, then its timely detection and treatment will not allow the development of an autoimmune process. After diagnosis, it is necessary to receive immunocorrective therapy with preservation and maintenance of functions internal organs.
It has been proven that with systemic connective tissue diseases, profound disturbances of immune homeostasis occur, expressed in the development autoimmune processes, that is, reactions immune system accompanied by the appearance of antibodies or sensitized lymphocytes directed against antigens own body(autoantigens).
 Treatment of systemic joint diseases
Treatment of systemic joint diseases
Among the methods of treating joint diseases are:
- medicinal;
- blockade;
- physiotherapeutic;
- therapeutic exercises;
- method of manual therapy;
- .
Medicines that are prescribed to a patient for arthrosis and arthritis have, for the most part, an effect that is aimed only at relieving pain symptom and inflammatory response. These are analgesics (including narcotics), non-steroidal anti-inflammatory drugs, corticosteroids, psychotropic drugs, and muscle relaxants. Ointments and rubs are often used for external use.
With the blockade method, the anesthetic device is injected directly into the source of pain - into trigger points in the joints, as well as into the places of the nerve plexuses.
As a result of physiotherapy, warming procedures reduce morning stiffness, ultrasound produces micro-massage of affected tissues, and electrical stimulation improves joint nutrition.
Joints affected by the disease need movement, so under the guidance of a doctor, you need to choose a program of physical therapy exercises and determine their intensity.
IN last years Manual therapy is popular in the treatment of joint diseases. It allows for a transition from forceful methods to soft, gentle ones, which are ideal for working with pathologically altered periarticular tissues. Manual therapy techniques involve reflex mechanisms, the impact of which improves metabolism in the affected elements of the joint and slows down degenerative processes in them. On the one hand, these techniques relieve pain (reduce unpleasant symptom diseases), on the other hand, promote regeneration, trigger recovery processes in a diseased organ.
Surgical treatment is indicated only in extremely advanced cases. However, before turning to surgery, it is worth thinking: firstly, surgical intervention is always a shock for the body, and secondly, sometimes arthrosis is precisely the consequence of unsuccessful operations.
Systemic connective tissue diseases are caused by the production of antibodies to own cells. This tissue is presented in bones, cartilage, and vessel walls. Even blood is a special type of it. The most common autoimmune diseases connective tissue - systemic lupus erythematosus and systemic scleroderma.
lupus erythematosus
Systemic lupus erythematosus usually affects women, and the disease debuts at a young age (15-25 years).
The exact cause of the disease is unknown. The influence of viral infections, stressful situations for the body (abortion, childbirth, severe mental trauma, excessive solar insolation), heredity and allergies is assumed.
The disease can begin acutely: fever, acute inflammation of the joints, skin) or gradually: a slight increase in temperature, joint pain, unmotivated weakness, weight loss.
Here are the symptoms of systemic connective tissue disease:
Redness of the nose and cheeks in the form of a “butterfly”;
Red ring-shaped rash;
Hyperemia of the skin in the décolleté area;
Ulcers in the lip area.
In addition, there is pain in the joints and muscles. Then they are amazed serous membranes heart, lungs, abdominal cavity, kidneys, liver, brain.
Systemic lupus erythematosus is often combined with antiphospholipid syndrome, which aggravates the course of the underlying disease.
Diagnosis is carried out on the basis of complaints, examination, laboratory research blood, urine and specific detection of antibodies. X-rays of the lungs, ultrasound of the abdominal cavity, and ECG are performed.
Treatment is prescribed by a rheumatologist, glucocorticosteroid hormones are used. In case of severe disease, immunosuppressants are added. Considering wide range side effects of these drugs require careful monitoring of the patient's condition. Plasmapheresis is also used. Patients suffering from a systemic disease are advised to follow a certain regimen: do not overcool, avoid the sun, surgical interventions, vaccinations.
Scleroderma
This systemic connective tissue disease is characterized by tissue damage when it thickens and hardens. As a rule, the disease occurs in women aged 30-40 years.
Its cause is also unknown; it is assumed that a genetic defect in the immune system plays a role, as well as viral infections, hypothermia and injury.
The disease debuts with the occurrence of pain in the fingers and disruption of the blood supply to them (Raynaud's syndrome). Lumps appear on the face and skin of the hands, followed by hardening. Then the skin of the neck, chest, legs, and feet becomes sclerotic. The face changes, it becomes mask-like. Movements in the joints become difficult. Later, the heart (shortness of breath, pain in the precordial region, swelling of the feet and legs) and the digestive system (difficulty swallowing, stool disorders) are affected.
Diagnosis is based on complaints, general examination, blood test results, and skin flap biopsy. To clarify damage to internal organs, ECG and echocardiography, radiography of joints, lungs, and FGDS are performed.
Treatment is prescribed by a rheumatologist: corticosteroid hormones, antifibrotic drugs, and immunosuppressants are used. As complementary therapy– physiotherapy and exercise therapy.
When autoimmune inflammation affects blood vessels, systemic vasculitis occurs. The following diseases of this group are distinguished:
Periarteritis nodosa – medium and small caliber arteries are affected;
Occurs, as a rule, in men. Characterized by muscle pain, fever, and weight loss. Abdominal pain, nausea, and vomiting are possible. Mental disorders and strokes may appear.
Giant cell temporal arteritis is a disease large vessels, mainly heads;
Typical for older people (60-80 years old). It manifests itself as weakness, severe pain, swelling in the temples, and a wave-like increase in temperature.
Takayasu's disease (nonspecific aortoarteritis) – inflammation of the walls of the aorta and large vessels;
Characterized by fainting, blurred vision, numbness and pain in the limbs, back, and abdomen.
Wegener's granulomatosis - affects the vessels of the respiratory system and kidneys;
Bloody and purulent nasal discharge, pain in the nasal area, ulcerative defects of the mucous membrane, destruction of the nasal septum, shortness of breath, hemoptysis, respiratory and renal failure occur.
Thromboangiitis obliterans - affects veins and arteries of the muscular type;
Due to damage to the blood vessels of the extremities, numbness and lameness develop.
Behcet's syndrome - manifested by stomatitis, damage to the eyes and mucous membrane of the genital organs.
The exact cause of vasculitis is not clear.
Diagnosis is carried out based on a combination of complaints, examination, results of blood tests, urine tests, and instrumental methods (angiography, chest x-ray).
For therapeutic purposes, glucocorticosteroid hormones, immunosuppressants and drugs that improve blood circulation are prescribed.
Patients with vasculitis require dynamic monitoring by a rheumatologist. ophthalmologist, cardiologist, nephrologist, neurologist, otorhinolaryngologist, surgeon, depending on the type of disease.
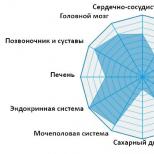
Systemic diseases are also called autoimmune or rheumatic. They differ from all others in that it is not a specific organ that is affected, but an entire system, and the cause of the damage is an attack of the immune system on the body’s own tissues, leading to their destruction.
Systemic diseases: list, symptoms and diagnosis
The main target for aggression is usually connective tissue, a large number of which is concentrated in joints and tendons, as well as in the skin and blood vessels, which is why they suffer the most in systemic diseases.
Systemic connective tissue diseases:
To system endocrine diseases relate:
- Hashimoto's thyroiditis;
- diffuse toxic goiter;
- Addison's disease;
- diabetes Type I
For systemic gastrointestinal diseases:
- nonspecific ulcerative colitis;
- Crohn's disease;
- primary biliary cirrhosis;
- Behçet's disease;
- Sjögren's disease (syndrome).
Systemic diseases also include autoimmune diseases of the heart, blood and kidneys, primary antiphospholipid syndrome, systemic vasculitis and some other pathologies.
All these diseases have different manifestations, for example, the symptoms of diabetes and psoriasis have little in common. However, there is a common feature - the involvement of the whole organism in the pathological state, the extensiveness (systematicity) of the lesions.
Systemic connective tissue diseases are characterized by persistent pain, most often localized in the muscles and joints. Often, a patient’s initial visit to the department of systemic diseases occurs due to skin lesions that are characteristic of SLE, scleroderma, psoriasis and some other pathologies.
Most systemic diseases are characterized by a chronic relapsing course with gradual progression. Without treatment, all these diseases lead to serious consequences - disability, and then death.
A comprehensive diagnosis of systemic diseases is carried out using laboratory and instrumental methods, and laboratory diagnostics in this case comes to the fore, making it possible to establish the autoimmune nature of the disease.
Immunodiagnostics includes determination of the following indicators:

Treatment of systemic diseases
Despite the incurable nature of systemic diseases, treatment is necessary - it allows you to stop the progression of the disease, eliminate painful symptoms, improve the quality of life and prolong it. With proper treatment, regular medical supervision and carrying out maintenance courses of therapy, remission can be very long (tens of years).
The following methods can be used to treat systemic diseases:
- Drug therapy. This is the main method. Treatment is carried out according to modern protocols: anti-inflammatory drugs, painkillers, promoting the restoration of connective tissue. If necessary (acute pain syndrome), a blockade is performed - injections of drugs directly into the lesion (joint).
- Physiotherapy. The method is used outside of exacerbations. Physiotherapy in various forms (massage, magnetic therapy, balneotherapy, electrophoresis, etc.) can improve the condition of the joint and muscle tissue, strengthen the walls of blood vessels.
- Physiotherapy - one of the most important methods when it comes to systemic diseases affecting joints and muscles. Dosed physical activity allows you to restore the functions of joints and muscles and stop degenerative processes in them.
- Surgery. The surgical method is used, as a rule, in the later stages of systemic diseases, when the functions of any organ are lost. For example, when a joint is destroyed, they resort to endoprosthetics - replacing the destroyed joint with an artificial one.
Autoimmune diseases are diseases associated with dysfunction of the human immune system, which begins to perceive its own tissues as foreign and damage them. Such diseases are also called systemic, because, as a rule, the entire system or even the entire body is affected.
Nowadays, we often talk about new infections that pose a threat to all of humanity. This is, first of all, AIDS, as well as SARS (atypical pneumonia), bird flu and other viral diseases. If we recall history, most dangerous viruses and bacteria were defeated, largely due to stimulation of one’s own immune system (vaccination).
The mechanism of occurrence of these processes has not yet been identified. Experts cannot understand what is causing negative reaction immune system on its own tissues. Injuries, stress, hypothermia, various infectious diseases, etc. can trigger a malfunction in the body.
Diagnosis and treatment of systemic diseases can be carried out by doctors such as a therapist, immunologist, rheumatologist and other specialists.
Examples
The most famous disease from this group is rheumatoid arthritis. However, this disease is by no means the most common autoimmune pathology. The most common autoimmune lesions of the thyroid gland are diffuse toxic goiter ( Graves' disease) and Hashimoto's thyroiditis. Type I diabetes mellitus, systemic lupus erythematosus and multiple sclerosis also develop through an autoimmune mechanism.
Not only diseases, but also some syndromes can have an autoimmune nature. A typical example is chlamydia, a disease caused by chlamydia and sexually transmitted. With this disease, the so-called Reiter's syndrome may develop, which is characterized by damage to the eyes, joints and genitourinary organs. These manifestations are not associated with direct exposure to the microbe, but arise as a result of autoimmune reactions.
Causes
In the process of maturation of the immune system, the main time of which falls on the period from birth to 13-15 years, lymphocytes - cells of the immune system - undergo “training” in the thymus and lymph nodes. In this case, each cell clone acquires the ability to recognize certain foreign proteins in order to fight various infections in the future.
Some lymphocytes learn to recognize the proteins of their body as foreign. Normally, such lymphocytes are tightly controlled by the immune system and probably serve to destroy defective or diseased cells of the body. However, in some people, control over these cells is lost, their activity increases and the process of destruction of normal cells begins - an autoimmune disease develops.
The causes of autoimmune diseases are not well understood, but existing information allows us to divide them into external And internal.
External causes are mainly pathogens of infectious diseases or physical impact, such as ultraviolet radiation or radiation. When a specific tissue is affected human body, they change their own molecules in such a way that the immune system perceives them as foreign. After an “attack” on the affected organ, the immune system causes chronic inflammation and, accordingly, further damage to its own tissues.
Another external cause is the development of cross immunity. This happens when the infectious agent turns out to be “similar” to its own cells - as a result, the immune system simultaneously attacks both the microbe and the cells (one explanation for Reiter's syndrome in chlamydia).
Internal reasons are, first of all, gene mutations, transmitted by inheritance.
Some mutations can change antigenic structure a certain organ or tissue, preventing lymphocytes from recognizing them as “their own” - such autoimmune diseases are called organ-specific. Then the disease itself will be inherited (the same organs will be affected in different generations).
Other mutations can disrupt the balance of the immune system by disrupting the control of self-aggressive lymphocytes. Then a person, when exposed to stimulating factors, can develop an organ-nonspecific autoimmune disease that affects many systems and organs.
Treatment. Promising methods
Treatment for autoimmune (systemic) diseases involves taking anti-inflammatory drugs and drugs that suppress the immune system (they are very toxic and such therapy contributes to susceptibility to various kinds infections).
Existing medications do not act on the cause of the disease, or even on the affected organ, but on the entire body. Scientists are striving to develop fundamentally new methods that will act locally.
The search for new drugs against autoimmune diseases follows three main paths.
Gene therapy seems to be the most promising method, with the help of which it will be possible to replace defective gene. However, before practical application gene therapy is still a long way off, and mutations corresponding to a specific disease have not been found in all cases.
If the cause turns out to be a loss of body control over the cells of the immune system, then some researchers suggest simply replacing them with new ones, first carrying out strict immunosuppressive therapy. This technique has already been tested and shown satisfactory results in the treatment of systemic lupus erythematosus and multiple sclerosis, but it is still unknown how long this effect lasts and whether suppression of the “old” immunity is safe for the body.
Perhaps, before others, methods will become available that do not eliminate the cause of the disease, but specifically remove its manifestations. These are, first of all, antibody-based drugs. They are able to block the immune system from attacking their own tissues.
Another way is to prescribe substances that take part in the fine regulation of the immune process. That is, we are not talking about substances that suppress the immune system as a whole, but about analogues of natural regulators that act only on certain types of cells.
Chapter 24. CONNECTIVE TISSUE DISEASES
Chapter 24. CONNECTIVE TISSUE DISEASES
Diffuse connective tissue diseases include rheumatoid arthritis, juvenile arthritis, systemic lupus erythematosus, scleroderma, dermatomyositis, Sjogren's syndrome. The most common are rheumatoid arthritis and systemic lupus erythematosus, the etiology of which is unknown.
24.1. RHEUMATOID ARTHRITIS
Rheumatoid arthritis is considered as a common chronic polyarthritis 1 with nonspecific inflammation of peripheral joints, usually symmetrical. Often, along with articular syndrome, it is noted systemic manifestations.
Epidemiology. The prevalence is about 1%. Women get sick 2-3 times more often than men.
Pathogenesis. In rheumatoid arthritis, two interrelated processes unfold in the joints: activation and proliferation of 2 immunocompetent cells (lymphocytes, macrophages) with the production of autoantibodies and the release of inflammatory mediators, as well as the proliferation of synovial membrane cells 3, which form aggressive granulation tissue - pannus, growing in the joint and destroying cartilage and subchondral bone. Activation of the immune system is considered the primary process that triggers the proliferation of synoviocytes.
In rheumatoid arthritis, capillaries grow into the cartilage, promoting pannus penetration and destruction. Pannus cells multiply, carry many adhesion molecules on their surface, secrete proteolytic enzymes and destroy nearby
1 Arthritis is inflammation of a joint, polyarthritis is inflammation of several joints.
2 Proliferation - tissue growth through the formation of new cells.
3 Synovial membrane is a connective tissue membrane that covers the outside of the joint.
tissues - cartilage and subchondral bone. Destruction of cartilage and subchondral bone leads to the formation of erosions of the articular surfaces, deformation of joints with subluxations, and then to ankylosation of 1 joints.
Symptom complex rheumatoid arthritis. The disease can begin acutely, with simultaneous damage to many joints, or (more often) progresses gradually.
The inflammatory process in the joint causes pain, swelling and limitation of movement. Stiffness and pain in the small joints of the hands and feet intensify in the morning, after a long stay in one position, and disappear with movement. Along with this, sleep disturbances, malaise, daytime weakness, increased fatigue, and weight loss appear.
Articular manifestations. The joints are deformed, the deformation of the interphalangeal joints is especially noticeable, they become spindle-shaped. Typical for rheumatoid arthritis is the deviation of the fingers to the ulnar side (ulnar deviation) and the simultaneous slipping of the extensor tendons from the metacarpophalangeal joints.
To systemic manifestations rheumatoid arthritis includes subcutaneous rheumatoid nodules (dense subcutaneous nodules in the area of bone protrusions, near joints and on extensor surfaces), vasculitis 2, pleural or pericardial effusion, Sjogren's syndrome (dryness of the mucous membrane of the mouth, eyes and other mucous membranes) .
Course and severity of the disease. The course of the disease is chronic; rheumatoid arthritis can begin at any age (usually at 25-50 years). The disease can lead to progressive destruction of both articular and extra-articular structures.
Diagnosis and examination methods. Diagnosis is based on the patient's complaints (joint pain and morning stiffness). Inspection of the affected joints (symmetrical lesions and ulnar deviation) is of great importance.
The main serological sign of rheumatoid arthritis is considered to be rheumatoid factor, which is detected in 80-90% of patients (seropositive rheumatoid arthritis) (Table 24-1).
1 Ankylosis is joint immobility caused by the development of fibrous, cartilaginous or bone adhesions between the articular surfaces of articulating bones.
2 Vasculitis is inflammation of blood vessels.
Typical radiological signs of rheumatoid arthritis:
Symmetrical increase in the volume of periarticular soft tissues;
Periarticular osteoporosis 1;
Narrowing of the joint space;
Marginal erosion of joints;
Absence of pronounced bone growths 2. The American Rheumatology Association has proposed clear diagnostic criteria rheumatoid arthritis. The diagnosis of rheumatoid arthritis is considered correct only when the patient has at least 4 criteria. The duration of existence of 1-4 criteria must be at least 6 weeks.
Diagnostic criteria for rheumatoid arthritis (American Rheumatological Association, 1987 revision):
Morning stiffness lasting at least 1 hour;
Arthritis of at least three joints;
Arthritis of the hand joints (wrist, metacarpophalangeal or proximal interphalangeal);
Symmetry of arthritis;
Rheumatoid nodules;
Detection of rheumatoid factor in blood serum by a method that gives no more than 5% positive results in the control group;
X-ray changes (changes in the hand - erosion or obvious periarticular osteoporosis).
24.2. JUVENILE RHEUMATOID ARTHRITIS
Juvenile rheumatoid arthritis develops before age 16 and is similar in many ways to adult rheumatoid arthritis. In juvenile rheumatoid arthritis, damage to one or more joints persists for 3 months or more. Oligoarthritis (50%) and polyarthritis (40%) are more often observed. In children younger age the disease occurs in a severe form (Still's syndrome), mainly with systemic manifestations.
1 Osteoporosis is a decrease in bone mineral density.
2 Marginal bone growths - osteophytes are typical for another common joint disease - osteoarthritis.
Epidemiology. The prevalence is 1 case per 1000 children.
Symptom complex of juvenile rheumatoid arthritis. The main manifestation (70%) is articular syndrome. However, unlike adult rheumatoid arthritis, pathological process Large joints are most often involved - knee, hip, ankle, wrist, elbow. In children, the pathological process involves cervical region spine and maxillotemporal joints, resulting in the development of micrognathia, which is pathognomonic for children - “bird jaw” with limited mouth opening. Involvement of the hip joints in the process affects the child’s posture (lordosis increases), and the gait becomes “duck-like.”
Simultaneously with articular syndrome, muscle atrophy develops, mainly proximal to the affected joint. Some children with severe disease (up to 30%) may experience extra-articular manifestations: long-term (weeks, months) febrile fever, mainly in the morning, skin rashes, enlarged lymph nodes, splenomegaly, heart damage (myocarditis, pericarditis), lung damage (pneumonitis), eye damage with a progressive decline in visual acuity up to complete blindness.
Diagnosis Juvenile rheumatoid arthritis is diagnosed based on the criteria listed below.
Diagnostic criteria for juvenile rheumatoid arthritis (American Rheumatological Association, 1987 revision):
Onset of disease before age 16;
Involvement of one or more joints with swelling/effusion or two of the following signs: limited function, pain on palpation, increased local temperature;
The duration of joint changes is at least 6 weeks;
Exclusion of all other rheumatic diseases.
24.3. SYSTEMIC LUPUS ERYTHEMATOSUS
Systemic lupus erythematosus is the most common diffuse connective tissue disease in adults. The main clinical manifestations are caused by vasculitis with predominant damage to small vessels. Up to 90% of patients with systemic lupus erythematosus complain
They affect joint manifestations from transient arthralgia to acute polyarthritis, sometimes occurring several years before the appearance of other symptoms.
Epidemiology. The prevalence is 1 case per 1000 population. The disease is more common in young women (90%) and children.
Pathogenesis. The pathological process develops mainly in the main substance of the connective tissue with damage to the basement membrane of the glomeruli of the kidney, skin, blood vessels, pleura, pericardium and endocardium.
Under the influence of a number of factors (increased insolation, focal infection, drugs, genetic factors) there is a deficiency of T-suppressors and a compensatory increase in the number of B-lymphocytes. Autoantigens to one’s own DNA are formed in the patient’s blood. As a result of the reaction of an autoantigen (own DNA) with autoantibodies, circulating immune complexes are formed, which are fixed on various organs and tissues of the body, causing immune inflammation (increased concentrations of prostaglandins, leukotrienes, complement). Autoimmune mechanisms contribute to self-maintenance and continuous progression of the pathological process.
The formation of immune complexes and their deposition on the basement membrane of blood vessels lead to widespread vasculitis and disruption of microcirculation in various organs and systems. As a result of fibrin deposition and microthrombosis of capillaries, arterioles and venules, DIC syndrome develops, which leads to ischemia and hemorrhages in organs. Morphologically, this is manifested by disorganization of connective tissue and vasculitis. Almost all organs and tissues are affected.
Symptom complex. The disease may begin suddenly with a fever that mimics an acute infection, or gradually over months or years with episodes of fever and general malaise. Changes in any organs and systems are possible.
Most common symptom systemic lupus erythematosus - arthritis (90%) with symmetrical damage to small and medium joints. Bone destruction usually does not occur. With prolonged arthritis, tendon contractures with secondary joint deformation are possible.
Erythema in the form of a “butterfly” appears on the skin in the cheekbone area.
Discoid skin changes and erythematous 1, dense maculopapular 2 rashes on exposed areas of the neck, upper chest and elbows, and ulcers on the mucous membranes are also possible. Recurrent pleurisy (dry or ex-sudative) and pericarditis are often noted. Generalized lymphadenopathy (enlarged lymph nodes) often develops in children and young patients; splenomegaly (enlarged spleen) is possible (10% of cases).
When the central nervous system is predominantly affected, headaches, personality changes, psychoses, and epileptic convulsions predominate. Kidney damage can be minor or, conversely, steadily progress (lupus nephritis), leading to death. Proteinuria is the most common condition.
Examination methods and diagnostics. Systemic lupus erythematosus can be suspected based on complaints and general examination data. Antibodies to DNA are specific for systemic lupus erythematosus. Detection of LE cells in the blood is a less specific sign of the disease (see Table 24-1). Of great importance are blood indicators that reflect the systemic inflammatory response - ESR and C-reactive protein. However, these indicators are nonspecific, they are not included in the diagnostic criteria, their indicators can increase with any inflammatory process.
At x-ray examination in patients with systemic lupus erythematosus, signs of joint erosion are not detected.
Currently, the criteria presented below are used in the diagnosis of systemic lupus erythematosus. The diagnosis is valid if any four criteria are present.
Diagnostic criteria for systemic lupus erythematosus (American Rheumatological Association, 1987 revision):
Rash in the area of the zygomatic arches;
Discoid rash;
Increased photosensitivity of the skin;
Mouth ulcers;
Arthritis;
Serositis;
Kidney damage;
Leukopenia less than 440 9 /l;
1 Erythema - redness of the skin.
2 Papule - a dense nodule of various sizes, rising above the skin.
Hemolytic anemia and/or thrombocytopenia 10040 9 /l;
Neurological disorders;
Antibodies to DNA or Le-cells;
Increased titer of antinuclear antibodies.
24.4. CLINICAL AND PHARMACOLOGICAL APPROACHES TO THE TREATMENT OF DIFFUSE CONNECTIVE TISSUE DISEASES
The basis for the treatment of rheumatoid arthritis and systemic lupus erythematosus is considered to be the combined administration of fast-acting anti-inflammatory drugs (usually NSAIDs, less often glucocorticoids) and one of the long-acting (basic) drugs.
Anti-inflammatory drugs have not only symptomatic, but also partly pathogenetic effects. The effectiveness of anti-inflammatory drugs appears within 1 day after prescription, but stops almost as quickly after discontinuation.
Basic drugs, compared to anti-inflammatory drugs, more deeply suppress the inflammatory process by inhibiting immune reactions and slow down destructive changes in the joints. However therapeutic effect develops slowly over several weeks or months.
The main goals of treatment for rheumatoid arthritis are:
Suppress inflammation of joints and other tissues;
Repair significant joint damage to reduce pain and improve function.
IN acute period diseases, when the pain is significant, bed rest is recommended for a short time. In mild cases, periodic rest in bed is sufficient. Local rest for the joint is provided by removable splints. As a rule, a normal nutritious diet is recommended.
Traditional basis drug treatment rheumatoid arthritis are NSAIDs.
In patients with severe pain syndrome On the first day of treatment, NSAIDs can be administered parenterally, and subsequently taken orally. Commonly used NSAIDs for local application in the form of ointments and gels (indomethacin, ketoprofen, diclo-
phenac) in the treatment of rheumatoid arthritis have only auxiliary value.
Glucocorticoids have a powerful and rapid anti-inflammatory effect. In addition, they have pronounced immunomodulatory activity. With long-term treatment, the effectiveness of glucocorticoids decreases; in addition, they are not able to prevent progressive destruction of the joints, and when discontinued in patients with active rheumatoid arthritis, a pronounced exacerbation occurs. ADRs of glucocorticoids during long-term use force them to be prescribed only in the absence of a therapeutic effect of NSAIDs and in patients with systemic manifestations of rheumatoid arthritis.
Rheumatoid nodules and mild sensory neuropathies in themselves are not an indication for glucocorticoids. Elderly patients (75-80 years old) with rheumatoid arthritis tend to tolerate low doses of prednisolone better than NSAIDs, which are more likely to cause gastric and duodenal ulcers at this age.
Glucocorticoids are contraindicated in:
Hypersensitivity;
Severe infections (except septic shock and tuberculous meningitis);
Immunization with live vaccines;
Chicken pox.
Relative contraindications to the prescription of glucocorticoids: peptic ulcer, hypertension, diabetes, glaucoma. With long-term use of small doses of glucocorticoids serious complications occur rarely and can be easily corrected even with continued use of drugs (calcium supplements for osteoporosis, omeprazole or ranitidine for erosive and ulcerative lesions of the stomach and duodenum).
In the treatment of rheumatoid arthritis, intra-articular administration of glucocorticoids is widely used, which avoids their systemic administration. The duration of the local therapeutic effect of these drugs depends on the type of drug. The most lasting effect is exerted by triamcinolone and betamethasone, methylprednisolone, hydrocortisone, which are administered every 7-14 days. In many cases, the therapeutic effect can depend on the individual response of the patient and last up to several months.
In children with juvenile rheumatoid arthritis, after injection of drugs into the knee joint, the effect in 40% of cases persists for 2 years or more.
Doses of drugs depend on the size of the affected joints.
Repeated administrations, if necessary, are done at intervals from several weeks to 3-4 months, depending on the degree and duration of the therapeutic effect.
If after two injections into the same joint there is no improvement, subsequent injections are not indicated.
Contraindications for intra-articular administration of glucocorticoids: infectious arthritis, severe joint destruction, significant osteoporosis.
Tolerance to intra-articular administration of glucocorticoids is usually good. NDR: pain, temporary exacerbation inflammatory process, infection, especially in patients receiving immunosuppressive therapy, local atrophy and depigmentation of the skin, degenerative changes in the joint, formation of fistula tracts, tendon ruptures, systemic effects.
The volume of glucocorticoids administered into large joint, should not exceed 2 ml, medium - 1 ml, small - 0.5 ml. After administration, it is necessary to ensure immobilization of the joint for 1-2 days. Glucocorticoids can be injected into no more than 3 joints at a time. The intervals between injections into the same joint should be as long as possible. It is not recommended to inject glucocorticoids into the joints, which are the main support of the body, more than 3 times a year. Glucocorticoids should not be injected directly into tendons.
Basic treatment. Slow-acting drugs, unlike fast-acting NSAIDs, slow down joint destruction, affect immune processes, remission periods, and, due to their cumulative properties, retain their effect for several months after discontinuation. These include methotrexate, gold compounds, penicillamine, hydroxychloroquine, sulfasalazine. As a rule, they are prescribed when NSAIDs are insufficiently effective after 3 or 4 months of treatment. With rapid progression of the disease, these drugs are prescribed at an earlier time.
Gold compounds usually prescribed in addition to NSAIDs if they do not significantly suppress joint inflammation. Their effect develops after 3-4 months from the start of treatment. When maximum improvement is achieved, the dose is gradually reduced. If the drug is discontinued with the onset of remission, then usually an exacerbation develops again after 3-6 months. If you continue to administer maintenance doses, the achieved improvement may last for several years.
The effectiveness of treating rheumatoid arthritis with gold preparations is comparable to the effectiveness of methotrexate. The advantage is considered to be the absence of significant immunosuppression and the development of intercurrent infections. However, gold preparations cause many ADRs that require discontinuation of the drug.
Gold preparations are contraindicated in cases of severe dysfunction of the liver, kidneys, pregnancy, as well as hematological disorders.
During treatment with gold preparations, it is necessary to do urine and blood tests (hemoglobin concentration, leukocyte count, leukocyte formula and platelet count). The studies are repeated in the 1st month before each injection of the drug, and then every 1-2 weeks.
Currently, a new direction in the treatment of rheumatoid arthritis is actively developing, the so-called biological therapy, which uses antibodies, receptors for cytokines and other immunological active drugs. Among them, the most promising methods involve blocking the activity of the inflammatory cytokines tumor necrosis factor (TNF) and interleukin-1 (IL-1) by administering monoclonal antibodies, cytokine antagonists or cytokine receptor antagonists. These include leflunomide, etanercept and infliximab.
There are various treatment regimens for rheumatoid arthritis. According to the oldest scheme, treatment begins with ensuring rest and prescribing NSAIDs; if there is no improvement, aminoquinoline drugs are added, then derivatives of 5-aminosalicylic acid or gold preparations, and then glucocorticoids and cytostatics. However, when using this regimen, patients begin to receive effective basic drugs late.
Modern scheme "step-down bridge" involves the combined administration of methotrexate, a gold drug, an aminoquinoline drug (hydroxychloroquine), glucocorticoids and cytostatics from the first days. Once the effect is achieved, the drugs are gradually discontinued.
Treatment tactics for systemic lupus erythematosus depend on the location and severity of the pathological process.
With a moderate or undulating process with fever, arthritis, pleurisy, pericarditis, headaches or rash, basic therapy should be minimal, and sometimes not required at all. For example, arthralgias respond well to NSAIDs. Acetylsalicylic acid can be used, especially if there is a tendency to develop thrombosis, but large doses of this drug for systemic lupus erythematosus can cause toxic damage to the liver.
In severe forms of systemic lupus erythematosus, glucocorticoids are used. The initial dose of prednisolone is: for hemolytic anemia - 60 mg/day, for thrombocytopenic purpura - 40-60 mg/day, for severe polyserositis - 20-60 mg/day, for kidney damage - 20-60 mg/day (in combination with immunosuppressants).
Improvement usually does not occur earlier than 4-12 weeks of treatment, and may not occur until the dose of glucocorticoids is reduced.
For active systemic lupus erythematosus or lupus nephritis, combination treatment (glucocorticoids + immunosuppressants) is indicated. The most commonly used are azathioprine at a dose of 2.5 mg/kg per day or cyclophosphamide at a dose of 2.5 mg/kg per day. Intermittent use of immunosuppressants is possible: for example, cyclophosphamide (500 mg) is administered intravenously at intervals depending on blood test data.
In acute vasculitis and severe lupus damage to the central nervous system and kidneys (lupus nephritis, neurolupus, rheumatoid vasculitis, systemic necrotizing vasculitis), pulse therapy with glucocorticoids (methylprednisolone at a dose of 1000 mg intravenously for 1 hour) is often used daily for 3 days in a row. At the same time, cyclophosphamide is administered intravenously. This treatment can be combined with plasmapheresis.
For systemic lupus erythematosus of any severity, when the inflammatory process can be suppressed, minimum maintenance doses of glucocorticoids or other drugs are selected, reducing the dose by no more than 10%. The intervals between dose reductions depend on how quickly initial clinical improvement is achieved. Treatment results are assessed by the dynamics of clinical symptoms and laboratory parameters.
A glucocorticoid for external use is selected taking into account the location and nature of the lesion, and the dosage form is also important. For systemic lupus erythematosus it is preferable
ointments of moderate activity and mild, gentle local action, which practically do not cause systemic adverse reactions (hydrocortisone 17-butyrate*, prednicarbate* 3, mometasone furoate*).
Pulse therapy
At diffuse diseases connective tissue, in particular rheumatoid arthritis, pulse therapy is used. Indications for its implementation are considered to be high disease activity, refractory to conventional treatment methods, and pronounced systemic manifestations (severe cutaneous vasculitis).
Pulse therapy consists of prescribing ultra-high doses of glucocorticoids to short term. Methylprednisolone is most often used, which is administered in the form of succinate in a dose of 1-2 g intravenously drip over 30-60 minutes, once a day for 3-5 days. The maximum concentration of the drug in the blood develops after 1 hour, followed by a decrease within 6-7 hours, but as a result of the non-genomic 1 mechanism of action, the effect is observed after a short period of time (several minutes). Methylprednisolone accumulates in various tissues, more in inflamed than in normal tissues, as well as in red blood cells. Pulse therapy allows you to achieve a quick effect and reduce maintenance doses of glucocorticoids for oral administration.
Classical pulse therapy for rheumatoid arthritis is rarely used; more often large doses of methylprednisolone (250-1000 mg) are administered intravenously in combination with cytostatics - methotrexate at a dose of 20 mg or cyclophosphamide at a dose of 400-1000 mg.
For systemic lupus erythematosus, along with the classical regimen of pulse therapy in elderly patients, especially with a tendency to hypertension and myocardial damage, modified regimens can be used (250-500 mg for 4-12 days).
In patients with the most severe forms of rheumatic diseases (lupus nephritis, lupus lesions of the central nervous system, rheumatoid vasculitis, systemic necrotizing vasculitis), pulse therapy must be combined with the use of cytostatics.
1 The main mechanism of action of glucocorticoids is to stimulate the transcription of certain genes, and its implementation requires at least 6-24 hours. Currently, the so-called non-genomic effects of these drugs, not related to the influence on the reading of genetic information of cells, are being studied.
24.5. CLINICAL PHARMACOLOGY OF NON-STEROID ANTI-INFLAMMATORY DRUGS
MEANS
NSAIDs are very widely used in clinical practice.
The great popularity of NSAIDs is explained by the fact that they, by providing anti-inflammatory, analgesic and antipyretic effects, bring relief to patients with symptoms (inflammation, pain, fever) that occur in many diseases.
NSAIDs are classified depending on the severity of anti-inflammatory activity and chemical structure (Table 24-2). Group 1 includes drugs with a pronounced anti-inflammatory effect. NSAIDs of group 2, which give a weak anti-inflammatory effect that has virtually no clinical significance, are often referred to as “non-narcotic analgesics” or “analgesics-antipyretics.”
Table 24-2. Classification of NSAIDs

From a practical point of view, it is important that drugs of the same group and even similar ones chemical structure differ somewhat both in the strength of the effect and in the frequency of development and nature of ADRs. The clinical effectiveness of the drug may depend on the type and characteristics of the disease in a particular patient, as well as on his individual reaction.
Pharmacokinetics
All NSAIDs are well absorbed from the gastrointestinal tract. To a large extent (more than 90%) bind to plasma albumin, displacing some other drugs and enhancing their effects. Many NSAIDs penetrate well into synovial fluid. NSAIDs undergo biotransformation in the liver, metabolites are excreted by the kidneys.
Pharmacodynamics
The main and general element of the mechanism of action of NSAIDs is considered to be inhibition of the synthesis of prostaglandins from arachidonic acid by inhibiting the enzyme cyclooxygenase (Fig. 24-1).

Rice. 24-1. Metabolism of arachidonic acid
Prostaglandins have versatile biological activity: act as mediators of the inflammatory reaction, sensitize receptors to pain mediators (histamine, bradykinin) and mechanical stress, lowering the threshold pain sensitivity, increase the sensitivity of the hypothalamic thermoregulation centers to the action of endogenous pyrogens (IL-1), the formation of which is induced by microorganisms and toxins.
Currently, two cyclooxygenase isoenzymes have been isolated that inhibit NSAIDs. The first (COX-1) controls the production of prostaglandins, which regulate the integrity of the gastrointestinal mucosa, platelet function and renal blood flow. The second (COX-2), involved in the synthesis of prostaglandins during inflammation, is intensively formed under the influence of a number of tissue factors that initiate the inflammatory response (cytokines). It is believed that the anti-inflammatory effect of NSAIDs is due to the inhibition of COX-2, and their unwanted reactions- inhibition of COX-1, and the drugs differ in selectivity in relation to various forms cyclooxygenase, which allows us to judge their comparative activity and toxicity.
Thus, pronounced selectivity for COX-1 is characteristic of acetylsalicylic acid, indomethacin, ketoprofen, piroxicam, and sulindac®. Diclofenac, ibuprofen, naproxen, lornoxicam exhibit moderate selectivity for COX-1; moderate selectivity for COX-2 is demonstrated by etodolac®, meloxicam, nimesulide, nabumetone®; pronounced selectivity for COX-2 is demonstrated by celecoxib.
The anti-inflammatory effect of NSAIDs may be associated with the stabilization of lysosome membranes, inhibition of neutrophil activation and impaired release of inflammatory mediators from them. In the implementation of the analgesic effect, disruption of the conduction of pain impulses at the level of the spinal cord (metamizole sodium) and activation of opioid receptors (lornoxicam) are important.
NSAIDs primarily suppress the exudation phase. In terms of anti-inflammatory activity, all NSAIDs are inferior to glucocorticoids, which, by inhibiting the enzyme phospholipase, inhibit the metabolism of phospholipids and disrupt the formation of prostaglandins and leukotrienes, also the most important mediators of inflammation.
The development of the anti-inflammatory effect lags behind the analgesic effect. The pain subsides in the first hours, and the anti-inflammatory effect occurs after 10-14 days regular intake, and when prescribing naproxen or oxicams even later - after 2-4 weeks.
Analgesic effect of NSAIDs manifests itself to a greater extent with pain of mild and moderate intensity in muscles, joints, tendons, nerve trunks, with headaches or toothaches. For severe visceral pain, most NSAIDs are less effective than narcotic analgesics (morphine group). Unlike narcotic analgesics, NSAIDs do not depress the respiratory center and do not cause drug dependence.
Antipyretic effect. NSAIDs can only reduce elevated temperature body and do not affect normal. Patients should be warned that NSAIDs have only symptomatic effects and have neither antibacterial nor antiviral activity. If fever, pain, or deterioration persist general condition patients should consult a doctor.
Anti-aggregation effect. As a result of inhibition of COX-1 in platelets, the synthesis of the endogenous proaggregant thromboxane is suppressed. Acetylsalicylic acid has the most pronounced antiaggregation activity, which is prescribed in a low daily dose (75-250 mg). Selective COX-2 inhibitors do not affect platelet aggregation.
Indications for the use of non-steroidal anti-inflammatory drugs
Rheumatic diseases. Rheumatism (rheumatic fever), rheumatoid, gouty and psoriatic arthritis, ankylosing spondylitis (Bechterew's disease), Reiter's syndrome.
Non-rheumatic diseases of the musculoskeletal system. Osteoarthritis, myositis, tendovaginitis, trauma (domestic, sports).
Neurological diseases. Neuralgia, radiculitis, sciatica, lumbago.
Renal, hepatic colic.
Headache, toothache, postoperative pain.
Fever (usually at a body temperature above 38.5 °C).
Prevention of arterial thrombosis.
Dysmenorrhea (relief of pain associated with increased uterine tone due to overproduction of prostaglandin F2a; in addition to the analgesic effect, blood loss is reduced).
The main negative property of NSAIDs is the high risk of developing adverse reactions from the gastrointestinal tract. 30-40% of patients receiving NSAIDs experience dyspeptic disorders (abdominal pain, nausea, vomiting), 10-20% have erosions and ulcers of the stomach and duodenum, and 2-5% have bleeding and perforation.
The ulcerogenic effect is partly due to the local damaging effects of NSAIDs and is mainly due to the inhibition of COX-1 as a result of systemic action. Clinical manifestations are absent in almost 60% of patients, especially in the elderly, and the diagnosis in many cases is made by endoscopy. Insignificant, but constantly bleeding erosions and ulcers can lead to systematic loss of blood in the stool (2-5 ml/day) and the development of iron deficiency anemia. Have slightly less gastrotoxicity dosage forms with enteric coating.
Risk factors for gastrotoxicity: female gender, age over 60 years, smoking, alcohol abuse, family history of peptic ulcers, concomitant severe cardiovascular diseases, taking glucocorticoids, immunosuppressants, anticoagulants, long-term treatment with NSAIDs, large doses or simultaneous use of two or more NSAIDs. Acetylsalicylic acid, indomethacin and piroxicam have the greatest gastrotoxicity.
Methods for improving the tolerability of non-steroidal anti-inflammatory drugs
Simultaneous administration of drugs that protect the gastrointestinal mucosa. According to controlled clinical studies, high efficiency has synthetic analogue prostaglandin E2 - misoprostol, which helps prevent the development of ulcers both in the stomach and in duodenum. The proton pump inhibitor omeprazole has approximately the same effectiveness as misoprostol, but is better tolerated.
Changing the tactics of using NSAIDs:
Dose reduction;
Switch to parenteral, rectal or local administration;
Prescription of enteric dosage forms; the negative effect on the gastrointestinal tract is not so much a local as a systemic reaction.
The use of NSAIDs that exhibit selectivity for COX-2, especially in patients at risk.
The development of a stomach ulcer in a patient requires discontinuation of NSAIDs and specific treatment. Continued use of NSAIDs, for example, for rheumatoid arthritis, is only possible with the prescription of misoprostol or omeprazole and regular endoscopic monitoring.
Two main mechanisms for the negative effects of NSAIDs on the kidneys have been identified:
By blocking the synthesis of prostaglandins in the kidneys, NSAIDs cause vasoconstriction and deterioration of renal blood flow; this leads to development ischemic changes in the kidneys and dysfunction; as a result, edema, hypernatremia, hyperkalemia occur, serum creatinine concentration and blood pressure increase; indomethacin and phenylbutazone have the most significant effect on renal blood flow;
Direct effect on the renal parenchyma with the development of interstitial nephritis (so-called analgesic nephropathy) and severe renal failure;
Risk factors for nephrotoxicity: age over 65 years, liver cirrhosis, previous renal pathology, decreased blood volume, long-term use of NSAIDs, concomitant use of diuretics.
Hematotoxicity is most typical for NSAIDs of the pyrazolidine and pyrazolones group, especially phenylbutazone. The most serious complications when using them are aplastic anemia and agranulocytosis. Phenylbutazone should be prescribed only as a reserve drug and, if possible, for a short course.
Hepatotoxicity consists mainly of changes in the activity of transaminases and other enzymes. In severe cases, jaundice and drug-induced hepatitis develop.
Hypersensitivity reactions - rash, Quincke's edema, anaphylactic shock, bronchospasm. The aspirin triad has been described: a combination of nasal and/or paranasal sinus polyposis, asthma and complete intolerance acetylsalicylic acid. It is advisable to avoid prescribing this drug to patients with bronchial asthma.
Neurotoxicity is manifested by headache, dizziness, impairment reflex reactions, most characteristic of indomethacin.
Reye's syndrome develops when acetylsalicylic acid is prescribed to children with viral infections (influenza, chicken pox). It manifests itself as severe encephalopathy, cerebral edema and liver damage without jaundice, but with a high concentration of cholesterol and increased activity of liver enzymes. Mortality is very high (up to 80%). Acetylsalicylic acid should not be used for viral infections in children under 12 years of age.
Contraindications to the use of non-steroidal anti-inflammatory drugs
NSAIDs are contraindicated for erosive and ulcerative lesions of the gastrointestinal tract, especially in the acute stage, severe impairment of liver and kidney function, cytopenias, individual intolerance, and pregnancy. If necessary, the safest use (but not before childbirth!) is small doses of acetylsalicylic acid.
Assignment Rules
For each patient, the most effective drug with the best tolerability should be selected. The sensitivity of patients to NSAIDs of even one chemical group can vary greatly, and the ineffectiveness of one of the drugs does not indicate the ineffectiveness of the group as a whole.
Treatment should begin with the lowest dose; if well tolerated, it can be increased after 2-3 days. In recent years, there has been a tendency to increase single and daily doses of well-tolerated drugs (ibuprofen) while maintaining restrictions for maximum doses acetylsalicylic acid, indomethacin, piroxicam. It should be taken into account that the anti-inflammatory effect of acetylsalicylic acid is manifested only in doses above 4 g/day.
For long-term treatment, NSAIDs should be taken after meals. To obtain a quick analgesic or antipyretic effect, it is preferable to prescribe the drugs on an empty stomach and drink a glass of water. NSAIDs are absorbed most quickly from the gastrointestinal tract and, therefore, give a faster effect [naproxen, diclofenac, water-soluble (effervescent) forms of acetylsalicylic acid and paracetamol]. To quickly relieve pain, parenteral dosage forms of NSAIDs (diclofenac, ketorolac) can also be prescribed.
The moment of taking NSAIDs can be determined by the maximum severity of the symptoms of the disease (pain, stiffness in the joints). You can deviate from generally accepted regimens (take 2-3 times a day), which usually allows you to achieve a greater therapeutic effect with a smaller daily dose.
The simultaneous use of two NSAIDs or more is inappropriate, since the effectiveness of such combinations has not been objectively proven (an exception may be the use of paracetamol in combination
with any other NSAID to enhance the analgesic effect) and the risk of adverse reactions increases.
Interaction with other drugs
When administered simultaneously, NSAIDs may enhance the effect of indirect anticoagulants and hypoglycemic agents. However, they weaken the effect of antihypertensive drugs, increase the toxicity of aminoglycosides, digoxin and some other drugs, which has a significant clinical significance(Table 24-3).
Many drugs prescribed concomitantly with NSAIDs, in turn, can affect their pharmacokinetics and pharmacodynamics:
Aluminum-containing antacids (Almagel*, Maalox*) and col-styramine ® reduce the absorption of NSAIDs in the gastrointestinal tract;
Glucocorticoids and slow-acting (basic) anti-inflammatory drugs (gold preparations) enhance the anti-inflammatory effect of NSAIDs;
Narcotic analgesics and sedatives enhance the analgesic effect of NSAIDs.
Acetylsalicylic acid(aspirin)
Acetylsalicylic acid is the first NSAID. When conducting clinical trials it typically serves as the standard against which others compare its effectiveness and tolerability
NSAIDs.
Pharmacodynamics
The pharmacodynamics of acetylsalicylic acid depends on daily dose: small doses - 30-325 mg - cause inhibition of platelet aggregation; medium doses (0.5-2 g) have an analgesic and antipyretic effect; large doses (4-6 g) have an anti-inflammatory effect. In high doses (more than 4 g) it stimulates excretion uric acid, disrupting its reabsorption in the renal tubules.
Pharmacokinetics
Well absorbed into gastrointestinal tract. The half-life of acetylsalicylic acid is only 15-20 minutes. Under the influence of esterases in the mucous membrane of the stomach, liver and blood from ace-


tylsalicylic acid, salicylate is cleaved off, which has the main pharmacological activity. The maximum concentration of salicylate in the blood develops 2 hours after taking acetylsalicylic acid, its half-life is 4-6 hours. Metabolized in the liver, excreted in the urine, and when the pH of the urine increases (for example, in the case of antacids), excretion increases. When using large doses of acetylsalicylic acid, it is possible to saturate metabolizing enzymes and increase the half-life of salicylate to 15-30 hours.
Interactions
Glucocorticoids accelerate the metabolism and excretion of acetylsalicylic acid. The absorption of acetylsalicylic acid in the gastrointestinal tract is enhanced by caffeine and metoclopramide. Acetylsalicylic acid inhibits gastric alcohol dehydrogenase, which leads to an increase in ethanol levels in the body.
NLR
Acetylsalicylic acid can cause damage to the gastric mucosa and lead to the development of erosions and/or ulcers, which are often complicated by bleeding, even when used in low doses - 75-300 mg / day (as an antiplatelet agent). The risk of bleeding is dose-related. Increased bleeding develops as a result of impaired platelet aggregation and inhibition of prothrombin synthesis in the liver (the latter with an aspirin dose of more than 5 g/day).
When taking acetylsalicylic acid, hypersensitivity reactions are possible: skin rash, bronchospasm. There is a special nosological form - Fernand-Vidal syndrome (“aspirin triad”): a combination of nasal and/or paranasal sinus polyposis, bronchial asthma and complete intolerance to acetylsalicylic acid. Therefore, acetylsalicylic acid and other NSAIDs are recommended to be used with great caution in patients with bronchial asthma.
Reye's syndrome develops when acetylsalicylic acid is prescribed to children with viral infections (influenza, chicken pox). It manifests itself as severe encephalopathy, cerebral edema and liver damage, which occurs without jaundice, but with high levels of cholesterol and liver enzymes. Gives a very high mortality rate (up to 80%). Therefore, acetylsalicylic acid should not be used for acute respiratory viral infections in children under the first 12 years of life.
Overdose or poisoning in mild cases is manifested by symptoms of “salicylicism”: tinnitus (a sign of “saturation” with salicylate), stupor, hearing loss, headache, visual disturbances, and sometimes nausea and vomiting. In severe intoxication, disorders of the central nervous system and water-electrolyte metabolism develop. Shortness of breath is noted (as a result of stimulation respiratory center), disturbances of the acid-base state (first respiratory alkalosis due to loss of carbon dioxide, then metabolic acidosis due to inhibition of tissue metabolism), polyuria, hyperthermia, dehydration. Oxygen consumption by the myocardium increases, heart failure and pulmonary edema may develop. Most sensitive to toxic effect salicylate in children under 5 years of age, in whom, just like in adults, it is manifested by severe acid-base disturbances and neurological symptoms.
Indications
Acetylsalicylic acid is used as an analgesic, antipyretic and antiplatelet agent. Acetylsalicylic acid is prescribed immediately if myocardial infarction is suspected or ischemic stroke. At the same time, acetylsalicylic acid has little effect on thrombus formation in the veins, so the drug should not be used for the prevention of postoperative thrombosis in surgery, where heparin is the drug of choice. Acetylsalicylic acid is one of the drugs of choice for the treatment of rheumatoid arthritis, including juvenile arthritis.
Diclofenac
Diclofenac is one of the widely used NSAIDs in the world. Diclofenac combines high anti-inflammatory activity with good tolerability during long-term use and is widely used in rheumatology. Has a strong and rapid analgesic effect.
Pharmacokinetics
Diclofenac is well absorbed from the gastrointestinal tract. Bioavailability is 50-60%, which is due to the “first pass” effect. The maximum concentration in the blood develops 0.5-2 hours after oral administration and 10-30 minutes after intramuscular administration. The half-life is 1.5-2 hours.
NLR
Diclofenac is generally well tolerated. At long-term use the drug may have a negative effect on the gastrointestinal tract and, especially, the liver, so clinical and laboratory monitoring is necessary.
Meloxicam
Meloxicam is a representative of a new generation of NSAIDs - selective COX-2 inhibitors. Thanks to this property, meloxicam selectively inhibits the formation of prostaglandins involved in the formation of inflammation. At the same time, it inhibits COX-1 much weaker, and therefore has less effect on the synthesis of prostaglandins, which regulate renal blood flow, the production of protective mucus in the stomach and platelet aggregation. The drug is often prescribed to patients with rheumatoid arthritis and osteoarthritis.
Pharmacokinetics
Bioavailability when taken orally is 89% and does not depend on food intake. The maximum concentration in the blood develops after 5-6 hours. Equilibrium concentration is created after 3-5 days. The half-life is 20 hours, which allows the drug to be administered once a day.
Ibuprofen
Ibuprofen, along with paracetamol, is one of the safest NSAIDs recommended for use, including in children. The drug is characterized by good analgesic and antipyretic effects, the anti-inflammatory activity of the drug is low. It is used more often as an analgesic, as well as in mild cases of rheumatoid arthritis and osteoarthritis.
Pharmacokinetics
The maximum concentration in the blood develops 1-2 hours after ingestion. It is quickly metabolized and excreted from the body. The half-life is 1.5-2.5 hours; therefore, the analgesic and antipyretic effects are maintained for up to 8 hours. The advantage of the drug is its good tolerability and the rare development of adverse reactions. It has the least damaging effect on the gastric mucosa among
other NSAIDs.
Metamizole
In Russia and some developing countries, metamizole (analgin) and metamizole-containing products are widely used. In the UK, Sweden, Norway, Saudi Arabia, UAE, USA, Australia, Israel, Denmark, the Netherlands, Ireland, Germany, Singapore and other countries, metamizole is prohibited for use due to a large number of adverse reactions, such as bone marrow suppression, agranulocytosis, aplastic anemia, development of complications from the kidneys (interstitial nephritis), liver (hepatitis), lungs (alveolitis), Lyell's and Stevens-Johnson syndromes.
Paracetamol (acetaminophen)
Paracetamol inhibits the synthesis of prostaglandins in the central nervous system than in peripheral tissues. Therefore, it has a predominantly “central” analgesic and antipyretic effect and has a very weak “peripheral” anti-inflammatory activity.
Pharmacokinetics
Paracetamol is well absorbed when taken orally and rectally. The maximum concentration in the blood develops 0.5-2 hours after administration. The drug is metabolized in the liver in 2 stages: first, under the influence of cytochrome P-450, intermediate hepatotoxic metabolites are formed, which are then conjugated with glutathione. 3% of administered paracetamol is excreted unchanged by the kidneys. The half-life is 2-2.5 hours. Duration of action - 3-4 hours.
Adverse reactions
Paracetamol is considered one of the safest NSAIDs. However, with long-term use of paracetamol, the risk of developing severe nephropathy leading to end-stage renal failure increases. It is based on the nephrotoxic effect of paracetamol metabolites, especially paraaminophenol. You should also be aware of the hepatotoxicity of paracetamol when taken in very large doses. A single dose of more than 10 g in adults or more than 140 mg/kg in children leads to poisoning, accompanied by severe liver damage. The reason is the depletion of glutathione reserves and the accumulation of intermediate products of paracetamol metabolism, which have a hepatotoxic effect.
It must be borne in mind that forced diuresis in case of paracetamol poisoning is ineffective and even dangerous; peritoneal dialysis and hemodialysis are ineffective. Taking sorbents, glutathione donors (acetylcysteine), and plasma transfusion are effective.
Interactions
The absorption of paracetamol in the gastrointestinal tract is enhanced by metoclopramide and caffeine.
Inducers of liver enzymes (phenytoin, barbiturates, rifampicin, phenylbutazone, tricyclic antidepressants, ethanol and some others) accelerate the breakdown of paracetamol to hepatotoxic metabolites and increase the risk of liver damage.
Nimesulide
Nimesulide is a selective COX-2 inhibitor. In this regard, it selectively disrupts the formation of prostaglandins involved in the formation of the inflammatory response, and does not affect the production of prostaglandins that regulate renal blood flow and the integrity of the gastrointestinal mucosa. Does not interfere with thromboxane synthesis, therefore does not affect platelet aggregation.
NLR
When using nimesulide, there is a fairly high risk of developing increased activity of hepatic transaminase, hepatitis, acute liver failure requiring liver transplantation. When taking nimesulide, allergic reactions are possible, including Stevens-Johnson syndrome and Lyell's syndrome. The use of nimesulide may adversely affect female fertility. The sale of nimesulide is prohibited in a number of countries. The European Medicines Agency (EMEA) has introduced a number of restrictions: the dosage regimen should not exceed 200 mg per day; the course of treatment should not exceed 15 days.
24.6. CLINICAL PHARMACOLOGY OF GLUCOCORTICOIDS
Glucocorticoids are hormones produced by the adrenal cortex. The term also refers to semi-synthetic drugs, such as prednisolone, dexamethasone and other drugs, which are derivatives of hydrocortisone, the most active natural glucocorticoid.
The adrenal cortex synthesizes two glucocorticoids: cortisone and hydrocortisone (cortisol). Cortisone is a biologically inactive compound that is converted to hydrocortisone in the liver. Both natural glucocorticoids have mineralocorticoid activity, but weaker than true mineralocorticoids.
The production of glucocorticoids is controlled by the hypothalamic-pituitary-adrenal system. The key organ regulating the synthesis of glucocorticoids is the hypothalamus, which responds to the concentration of hydrocortisone in plasma in the blood and stress. When there is a low concentration of glucocorticoids in the blood or stress (trauma, infection, physical stress), the hypothalamus produces corticotropin-releasing factor (corticoliberin), which stimulates the release of adrenocorticotropic hormone (ACTH) from the pituitary gland. Under the influence of ACTH, glucocorticoids and mineralocorticoids are synthesized in the adrenal glands. With an excess of glucocorticoids in the blood, the hypothalamus stops producing corticotropin-releasing factor. Thus, the hypothalamic-pituitary-adrenal system functions according to a negative feedback mechanism (Fig. 24-2).

Rice. 24-2. Regulation of the function of the hypothalamic-pituitary-adrenal system
The release of glucocorticoids from the adrenal glands into the blood during the day does not occur evenly, but in the form of 8-12 impulses, which obey the circadian rhythm. Maximum secretion of hydrocortisone occurs in the early hours (6-8 hours) and decreases sharply in the evening and at night.
Pharmacokinetics
Glucocorticoids are well absorbed from the gastrointestinal tract. The maximum concentration in the blood is observed after 0.5-1.5 hours. Food somewhat slows down the rate of absorption, but does not reduce its degree.
Glucocorticoids for injection are available in the form of various esters. Succinates, hemisuccinates and phosphates are water-soluble and have a rapid and relatively short-term effect. In emergency situations, these are the drugs of choice and are administered intravenously. When administered intramuscularly, the maximum effect develops after 1-2 hours. Acetates and acetonides are fine-crystalline suspensions, insoluble in water, the effect of which develops slowly (several hours) and lasts a long time (several weeks). They are intended for intra- and periarticular administration. When administered intramuscularly, they are slowly absorbed with the onset of action after 1-2 days, a maximum after 4-8 days and a duration of up to 4 weeks. They cannot be administered intravenously.
Metabolism. Glucocorticoids undergo biotransformation in the liver with the formation of inactive metabolites, and natural ones faster than semi-synthetic ones. Cortisone and prednisone first undergo first-pass metabolism into active forms- hydrocortisone and prednisolone, respectively. Fluorinated glucocorticoids (triamcinolone, dexamethasone, betamethasone) are biotransformed more slowly than all others.
In the blood plasma, glucocorticoids bind to proteins (trans-cortin, albumin), 90% natural, and 40-60% semi-synthetic. This is due to the higher concentration of semisynthetic glucocorticoids in tissues and their higher activity.
Excretion of inactive glucocorticoid metabolites is carried out by the kidneys. Natural glucocorticoids have the shortest T1/2, fluorinated drugs have the longest. In case of renal failure, this parameter does not change and no dose adjustment is required.
Pharmacodynamics
After passing through the cell membrane, glucocorticoids in the cytoplasm bind to a specific steroid receptor. The activated glucocorticoid-receptor complex penetrates the cell nucleus, binds to DNA and stimulates the formation of messenger RNA. As a result of RNA translation, various regulatory proteins are synthesized on ribosomes. One of the most important is lipocortin, which inhibits the enzyme phospholipase A 2 and thereby suppresses the synthesis of prostaglandins and leukotrienes, which are of great importance in the development of the inflammatory reaction.
There is also a theory of non-genomic action of glucocorticoids, according to which the effects are realized not only through the nucleus, but through membrane and cytoplasmic receptors. As a result, some glucocorticoid effects may occur more quickly, especially when high doses are administered intravenously. However, the maximum pharmacological activity glucocorticoids occur at a time when their peak concentrations in the blood are already behind them.
Water and electrolyte balance. Drugs in this group slow down the excretion of sodium and water from the body as a result of increased reabsorption in the distal renal tubules, and increase the excretion of potassium (mineralocorticoid activity). These effects are more characteristic of natural glucocorticosteroids (cortisone and hydrocortisone), less so of semi-synthetic ones (prednisone, prednisolone, methylprednisolone). The fluorinated drugs triamcinolone, dexamethasone and betamethasone do not have mineralocorticoid activity.
Carbohydrate metabolism. An increase in blood glucose concentration due to stimulation of gluconeogenesis in the liver, a decrease in membrane permeability to glucose. Glucosuria and steroid diabetes may develop.
Protein metabolism. Inhibition of synthesis and increased protein breakdown processes, especially in skin, muscle and bone tissue. This is manifested by weight loss, muscle weakness, skin and muscle atrophy, stretch marks, hemorrhages, and slow wound healing.
Fat metabolism. Redistribution of subcutaneous fat according to the Cushingoid type (Itsenko-Cushing syndrome: moon-shaped face, pituitary-type obesity, hirsutism, increased blood pressure, dysmenorrhea, stretch marks). This is due to the fact that lipolysis predominates in the tissues of the extremities, and lipogenesis predominates in the tissues of the chest, neck, face, and shoulder girdle.
Calcium metabolism. Glucocorticoids reduce calcium absorption in the intestine, promote its release from bone tissue and increase excretion in the urine. As a result, osteoporosis, hypocalcemia and hypercalciuria may develop.
The cardiovascular system. Glucocorticoids increase the sensitivity of adrenergic receptors to catecholamines and enhance the pressor effect of angiotensin II. They reduce capillary permeability, maintain normal arteriolar tone and myocardial contractility. With adrenal insufficiency it decreases cardiac output, arterioles dilate, the reaction to adrenaline weakens. Together with hypovolemia caused by mineralocorticoid deficiency, these changes can lead to vascular collapse.
Anti-inflammatory effect. Glucocorticoids inhibit all phases of inflammation. Many factors are important in their anti-inflammatory effect: inhibition of phospholipase A and the associated disruption of the formation of prostaglandins and leukotrienes, stabilization of lysosome membranes, reduction of capillary permeability, inhibition of the migration of neutrophils and macrophages to the site of inflammation, inhibition of fibroblast proliferation and collagen synthesis, suppression of cytokine formation by lymphocytes and macrophages.
Immunomodulatory and antiallergic effects. Glucocorticoids inhibit the proliferation of lymphoid tissue and cellular immunity, which underlies their use in organ and tissue transplantation. These drugs inhibit the formation and disrupt the kinetics of T-lymphocytes, reducing their cytotoxic activity, and prevent the interaction of immunoglobulins with mast cells and macrophages, inhibiting the release of biologically active substances from them.
Blood. Glucocorticoids cause lymphocytopenia, monocytopenia and eosinopenia, but stimulate the formation of red blood cells and platelets.
After taking even 1 dose of glucocorticoids, the number of lymphocytes, monocytes, eosinophils, and basophils decreases with the simultaneous development of neutrophilic leukocytosis. The maximum changes in the blood are observed after 4-6 hours, the initial state is restored after 24 hours. After completing a long course of glucocorticoids, changes in the blood can persist for 1-4 weeks.
Endocrine system. The administration of glucocorticoids is accompanied by inhibition of the hypothalamic-pituitary-adrenal system, which is due to negative feedback. Suppression is more pronounced with long-term use of glucocorticoids and/or prescription of long-acting drugs.
Glucocorticoids reduce the production of sex hormones as a result of direct suppression of their synthesis and a decrease in the production of luteinizing hormone by the pituitary gland.
Adverse drug reactions
With systemic administration of glucocorticoids, a wide variety of ADRs can develop (Table 24-4). The risk of their occurrence, as a rule, increases with increasing doses and duration of drug use.

Immunity _I Intensification of tuberculosis and other infections
Modern methods of using glucocorticoids (for example, alternating therapy), inhalation and intra-articular administration can not only reduce the incidence of ADRs, but also increase the effectiveness of treatment. However, with any treatment it is necessary to monitor the development of ADRs (monitoring body weight, blood pressure, electrolyte composition of the blood, the state of the gastrointestinal tract, musculoskeletal system, visual organs, determining the concentration of glucose in the blood and urine, monitoring the development of infectious complications).
Bacterial infections (usually in the form of pneumonia or septicemia) occur most frequently. The main pathogens are staphylococci and gram-negative bacteria intestinal group, which should be taken into account when choosing empirical antibacterial therapy.
Tuberculosis. Patients with positive tuberculin tests are at risk of developing severe tuberculosis, and with long-term treatment with glucocorticoids they should for preventive purposes take isoniazid.
Viral infections. The use of glucocorticoids increases the risk of dissemination of viral infections. In case of contact with a patient with chickenpox or herpes zoster, a patient who has not previously been ill should receive specific immunoglobulin within 48 hours. If the course of glucocorticoids exceeds 2 weeks, then the use of live viral vaccines is not recommended.
Secondary adrenal insufficiency. To the most severe complications Taking glucocorticoids, potentially life-threatening, includes secondary adrenal insufficiency - a consequence of inhibition of the hypothalamic-pituitary-adrenal system with long-term use of glucocorticoids.
Risk factors for oppression
hypothalamic-pituitary-adrenal system
Dose. When taking glucocorticoids in physiological doses (for an adult 2.5-5 mg/day prednisolone or 10-30 mg/day hydrocortisone) suppression of the hypothalamic-pituitary-adrenal
the system is not happening. At higher doses, dysfunction of the adrenal cortex is noted after 1-2 weeks, and subsequently its atrophy may develop.
Duration of treatment. With a course of up to 10 days (at a dose of no more than 40 mg/day of prednisolone), there is no danger of significant inhibition of the hypothalamic-pituitary-adrenal system; when taken for several weeks, atrophy of the adrenal cortex is possible.
Time of receipt. It is necessary to take into account the circadian rhythm of glucocorticoid production (it is more dangerous to take 5 mg of prednisolone in the evening than 20 mg in the morning).
Type of drug. Suppression of the hypothalamic-pituitary-adrenal system is more pronounced when taking fluorinated glucocorticoids - triamcinolone, dexamethasone, betamethasone with the longest action.
Withdrawal syndrome clinic. The severity of the withdrawal syndrome depends on the preservation of the function of the adrenal cortex. In mild cases appear general weakness, fatigue, loss of appetite, muscle pain, exacerbation of the underlying disease, increased temperature. In severe cases (especially with severe stress) a classic Addisonian crisis with vomiting, collapse, and convulsions may develop. Without the administration of glucocorticoids, patients quickly die from acute cardiovascular failure.
Measures to prevent secondary adrenal insufficiency:
With the exception of emergency conditions and special indications, it is recommended to prescribe glucocorticoids in accordance with the circadian rhythm;
It is necessary to use alternating therapy as widely as possible;
When the course of treatment lasts more than 10 days, glucocorticoids are discontinued with a gradual reduction in their dose; the withdrawal mode depends on the duration of use; for a course of several weeks to several months, it is permissible to reduce the dose by 2.5-5 mg of prednisolone (or an equivalent amount of another drug) every 3-5 days. With longer use, it is necessary to reduce the dose more slowly - by 2.5 mg every 1-3 weeks;
After discontinuation of glucocorticoids taken for 2 weeks or more, monitor the patient’s condition for 1.5-2 years. stressful situations. If necessary, provide protective therapy with glucocorticoids.
Interactions with other drugs
The effect of glucocorticoids is enhanced by concomitant administration of erythromycin (slows down the metabolism of glucocorticoids in the liver), salicylates (increases the non-protein-bound fraction of glucocorticoids), and estrogens.
The effect of glucocorticoids is weakened by inducers of microsomal liver enzymes - phenobarbital, phenytoin, rifampicin.
Glucocorticoids weaken the effect of anticoagulants, antidiabetic and antihypertensive drugs.
Glucocorticoids enhance the effect of theophylline, sympathomimetics, immunosuppressants, and NSAIDs.
Indications for the use of glucocorticoids
There are three fundamentally different regimens for prescribing glucocorticoids.
Replacement therapy. The use of glucocorticoids in physiological doses for adrenal insufficiency of any etiology. Cortisone or hydrocortisone is administered taking into account the circadian rhythm - 2/3 doses in the morning and 1/3 in the evening. Other drugs are prescribed once a day in the morning.
Suppressive therapy. The use of glucocorticoids for adrenogenital syndrome 1 in pharmacological (exceeding physiological) doses, which leads to suppression of ACTH secretion and a subsequent decrease in hypersecretion of androgens by the adrenal cortex; 1/3 of the daily dose of cortisone or hydrocortisone is usually given in the morning, and 2/3 of the dose in the evening.
Pharmacodynamic therapy. The most common use of glucocorticoids is divided into systemic and local. In systemic therapy, glucocorticoids are prescribed based on their anti-inflammatory, antiallergic, immunosuppressive and anti-shock effects. With systemic pharmacodynamic therapy, various routes of administration and dosage regimens of drugs can be used depending on the severity of the patient’s condition (Table 24-5). The most preferred drugs with an average duration of action are prednisone, prednisolone, methylprednisolone (Table 24-6).
1 Adrenogenital syndrome is associated with hypersecretion of the adrenal glands and sex hormones.

Table 24-6. Comparative activity of glucocorticoids

Drugs long acting should be prescribed in a short course. Dexamethasone has some special indications for use: bacterial meningitis, cerebral edema, prevention of respiratory distress syndrome in premature newborns (dexamethasone stimulates the synthesis of surfactant in the alveoli of the lungs), leukemia (replacing prednisolone with dexamethasone in acute lymphoblastic leukemia significantly reduces the incidence of central nervous system damage).
Principles of long-term treatment
It is preferable to use glucocorticoids with an intermediate duration of action.
Individual dose selection is necessary, depending more on the nature of the disease and the patient's response to treatment than on age or body weight.
The dose is reduced gradually to the minimum that ensures clinical stability after obtaining the desired effect.
Taking into account the physiological circadian rhythm of glucocorticoid secretion: in most cases, drugs should be prescribed in the form of one morning dose, it is possible to prescribe 2/3-3/4 doses in the morning, and the rest - around noon. This dosage regimen reduces the risk of suppression of the hypothalamic-pituitary-adrenal system, since in the morning this system is least sensitive to the suppressive effect of exogenous glucocorticoids.
Transfer of the patient to alternative therapy is possible only when the condition has stabilized.
Alternative therapy
Alternative therapy consists of prescribing a glucocorticoid every other day in the form of 1 dose, which should be 2 times greater than that administered before switching to alternative therapy.
The main advantage of this method is less suppression of the hypothalamic-pituitary-adrenal axis and, therefore, a reduced risk of developing adrenal insufficiency.
The patient is transferred to alternating therapy gradually and only after the condition has stabilized. For this prescription regimen, only glucocorticoids with an average duration of action (prednisolone, methylprednisolone, prednisone) are suitable, after taking 1 dose of which the hypothalamic-pituitary-adrenal system is suppressed for 12-36 hours. Long-acting drugs (triamcinolone, dexamethasone, betamethasone) cannot be used, so as when they are prescribed, even every other day, the risk of suppression of the hypothalamic-pituitary-adrenal system does not decrease.
Alternative therapy is not effective enough in the treatment of hematological diseases, ulcerative colitis, malignant tumors, and in severe conditions.
Pulse therapy
Pulse therapy consists of short-term administration of ultra-high doses of glucocorticoids. The minimal mineralocorticoid effect of methylprednisolone, its weaker effect on the gastrointestinal tract and central nervous system than prednisolone, make it the drug of choice when performing pulse therapy. Typically, methylprednisolone is administered at a dose of 1-2 g/day intravenously once a day for 3-5 days.
Indications for pulse therapy are severe and life-threatening diseases, primarily systemic collagenoses (systemic
lupus erythematosus, vasculitis, severe rheumatoid arthritis with visceral lesions, severe ankylosing spondylitis). Pulse therapy is also used for thrombocytopenic purpura, acute spinal cord injury, and multiple sclerosis.
In patients with the most severe forms of rheumatic diseases (lupus nephritis, lupus lesions of the central nervous system, rheumatoid vasculitis, systemic necrotizing vasculitis), pulse therapy should be combined with the use of cytostatics (cyclophosphamide).
Contraindications for the use of glucocorticoids are relative and should be taken into account when planning long-term treatment:
Diabetes (fluorinated glucocorticoids are especially dangerous);
Mental illnesses, epilepsy;
Peptic ulcer of the stomach and duodenum;
Severe osteoporosis;
Severe hypertension.
In emergency situations, glucocorticoids are administered without taking into account contraindications.
Glucocorticoids penetrate well through the placenta. Natural and non-fluorinated semi-synthetic drugs are generally safe for the fetus and do not lead to intrauterine development Cushing's syndrome and suppression of the hypothalamic-pituitary-adrenal system. Fluorinated glucocorticoids, when taken for a long time, can cause unwanted reactions, including deformities.
Glucocorticoids are used to prevent respiratory distress syndrome in premature infants. As a rule, long-acting drugs are prescribed, most often dexamethasone. It is administered intramuscularly to the mother during pregnancy up to 34 weeks, 24-48 hours before the expected birth.
A woman in labor who has been taking glucocorticoids for the last 1.5-2 years should additionally administer hydrocortisone hemisuccinate* 100 mg every 6 hours to prevent acute adrenal insufficiency.
When breastfeeding, low doses of glucocorticoids, equivalent to 5 mg of prednisolone, do not pose a danger to the baby due to poor penetration into breast milk. Higher doses of drugs and their long-term use can cause growth retardation and depression of the child’s hypothalamic-pituitary-adrenal system.
Local application of glucocorticoids
Local use of glucocorticoids makes it possible to create a high concentration of the drug in the pathological focus and significantly reduce the risk of developing undesirable systemic reactions. Topical options:
Inhalation (into the lungs or nasal cavity);
Intra-articular, periarticular;
Intradermal (into scars);
Epidural;
Intracavitary (intrapericardial, intrapleural);
Rectal;
External (skin, eyes, ears).
Intra-articular injection. For intra- and periarticular administration, water-insoluble injectable preparations are used. This creates a high concentration of glucocorticoids in the synovium and synovial fluid, ensuring maximum local anti-inflammatory effect with a minimum likelihood of systemic effects.
Indications for intra-articular administration. Rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Reiter's disease. Intra-articular administration is used for mono- or oligoarthritis, and in the case of polyarthritis - for severe inflammation of one or more joints.
The duration of the effect depends on the type of drug used and ranges from 1 to 3 weeks. In many cases, the therapeutic effect can depend on the individual response of the patient and last up to several months.
Contraindications. Infectious arthritis, severe joint destruction, significant osteoporosis, intra-articular fracture, periarticular cellulitis, osteomyelitis, bacterial endocarditis, sepsis, blood coagulation pathology.
Periarticular administration of glucocorticoids preferably for persistent inflammatory diseases of the periarticular tissues that cannot be treated with other drugs, with pain and dysfunction of the joints.
Indications. Capsulitis, tendovaginitis, bursitis, epicondylitis, plantar fasciitis, forearm tunnel syndrome.
For periarticular administration, it is advisable to use hydrocortisone acetate* (5-25 mg), since its action is shorter and negative.
The significant effect on connective tissue metabolism (impaired protein synthesis) is less pronounced than that of other drugs.
NLR. Pain, temporary exacerbation of the inflammatory process, infection, local atrophy and depigmentation of the skin, degenerative changes in the joint, aseptic necrosis bones, formation of fistula tracts (if crystals of the drug remain along the needle), damage to tendons or nerve trunks.
Prevention of ADRs. Strict adherence to asepsis and antisepsis, the use of a thin needle, local anesthetics, rest for the joint for 1-2 days after the procedure, simultaneous injection into no more than 3 joints, the longest possible intervals between injections into the same joint.
Cortisone- a natural glucocorticoid preparation, biologically inactive. Activated in the liver, turning into hydrocortisone. Has a short-term effect. Mainly used for replacement therapy of adrenal insufficiency in patients with normal liver function.
Prednisolone a synthetic glucocorticoid, most often used in clinical practice and considered as a standard drug. Refers to glucocorticoids with an average duration of action.
Methylprednisolone Compared to prednisolone, it has slightly greater (20%) glucocorticoid activity, minimal mineralocorticoid effect, and is less likely to cause undesirable reactions (especially changes in the psyche, appetite, ulcerogenic effect). Preferred when performing pulse therapy.
Dexamethasone is a fluorinated homolog of hydrocortisone. One of the most powerful glucocorticoids: 7 times stronger than prednisolone in glucocorticoid activity. Does not have mineralocorticoid effect. Causes severe depression of the hypothalamic-pituitary-adrenal system, severe disturbances in carbohydrate, fat, calcium metabolism, psychostimulant effect, therefore it is not recommended to prescribe it for long term. The drug has some special indications for use: bacterial meningitis; cerebral edema; in ophthalmology (keratitis, uveitis and others); prevention and treatment of nausea and vomiting during chemotherapy; treatment of severe withdrawal syndrome in alcoholism; prevention of respiratory distress syndrome in premature infants (dexamethasone stimulates the synthesis of surfactant in the alveoli of the lungs); leukemia (replacement of prednisolone with dexamethasone for
acute lymphoblastic leukemia significantly reduces the incidence of damage to the central nervous system).
24.7. CLINICAL PHARMACOLOGY OF DRUGS FOR BASIC THERAPY OF RHEUMATOID ARTHRITIS
Gold compounds
Gold compounds are usually prescribed in addition to NSAIDs if they do not significantly suppress joint inflammation. Sodium aurothiomalate* 3 and aurothioglucose* 3 are administered parenterally.
Gold preparations affect T-lymphocytes, disrupting their activation and the development of an autoimmune reaction; they are administered intramuscularly once a week.
The effectiveness of treating rheumatoid arthritis with gold preparations is comparable to methotrexate. The advantage is considered to be the absence of significant immunosuppression and a lower risk of developing intercurrent infections. However, gold preparations cause many ADRs that require their discontinuation.
Gold preparations are contraindicated in cases of severe dysfunction of the liver, kidneys, pregnancy, as well as hematological diseases.
During treatment with gold preparations, it is necessary to monitor the composition of urine, hemoglobin concentration, leukocyte count, leukocyte formula and platelet count. These studies are repeated for a month before each injection of the drug, and then every 1-2 weeks.
NLR. Itching, dermatitis, stomatitis, proteinuria, agranulocytosis, thrombocytopenia, aplastic anemia, diarrhea, hepatitis, pneumonitis.
If any ADR develops, treatment with gold preparations is interrupted. If the manifestations of ADR are mild (mild itching or isolated skin rashes), treatment can be carefully resumed after 2 weeks.
In case of significant complications, use dimercaprol (a drug that binds gold) at a dose of 2.5 mg/kg intramuscularly up to 4-6 times a day for the first 2 days, and then 2 times a day for 5-7 days.
When taking aurothiomalate* 3, especially if stored in the light, a short-term reaction is possible: flushing of the face, tachycardia, fainting a few minutes after administration. In such cases
it is necessary to switch to the use of another gold compound - aurothioglucose *, which does not cause similar reactions. A gold preparation for oral administration is auranofin®.
Penicillamine
If gold preparations are poorly tolerated or insufficiently effective, penicillamine is prescribed, which is significantly inferior to them in effectiveness and tolerability.
ADRs (up to 40%) that force one to stop treatment with penicillamine are noted more often than with treatment with gold. Penicillamine can inhibit bone marrow hematopoiesis and cause proteinuria, nephrotic syndrome, cholestatic jaundice and other serious complications (myasthenia gravis, pemphigus, Goodpasture's syndrome, polymyositis, lupus-like syndrome), as well as skin rashes and taste disorders. The appearance of the first signs of these complications requires cessation of treatment. An exception is a taste disorder, which may resolve spontaneously. Before starting treatment and every 2-4 weeks while taking the drug, you need to do a urine test and a blood test with a platelet count.
In children, due to significant adverse events, gold and penicillamine preparations are not widely used.
Derivatives of 5-aminosalicylic acid
Drugs from the sulfonamide group (sulfasalazine, mesalazine), which are used in the treatment of necrotizing ulcerative enterocolitis, are also prescribed for connective tissue diseases (rheumatoid arthritis). They are not inferior in effectiveness to penicillamine, but are superior to it in tolerability.
The action of the drugs is associated with antagonism towards folic acid and anticytokine effects similar to methotrexate.
NLR. Nausea, vomiting, neutropenia, hemolysis, hepatitis and skin rashes.
There are no significant differences between sulfasalazine and mesalazine in terms of effectiveness and tolerability. However, some patients tolerate one of these drugs better than the other.
Aminoquinoline drugs
Antimalarial drugs (chloroquine, hydroxychloroquine) are often used due to their good tolerability, but
Indeed, these are the weakest among the basic treatments for systemic connective tissue diseases.
The effect of the drugs is due to weak cytotoxic properties and inhibition of macrophage function. They can be used in patients with minimal manifestations of articular syndrome, since the effect develops slowly, after 3-6 months of continuous use.
ADRs are minor and rare: dermatitis, myopathy and corneal opacification, usually reversible. At the first complaints about vision, the drug is discontinued. Hydroxychloroquine is better tolerated.
24.8. CLINICAL PHARMACOLOGY
CYTOSTATICS AND IMMUNOSUPPRESSIVE DRUGS
Many drugs used for chemotherapy malignant neoplasms, have an immunosuppressive effect and can be used in transplantology and the treatment of autoimmune diseases. Cytostatics share a similar mechanism of action and the ability to block both B- and T-cell clonal activation. The most widely used are azathioprine, mycophenolate mofetil, cyclophosphamide, and methotrexate. Other antitumor cytostatics, such as chlorambucil, vincristine, vinblastine, dactinomycin, are not prescribed as immunosuppressive drugs.
Older cytostatics (azathioprine, cyclophosphamide, methotrexate) are characterized by an effect on the cells of many tissues and organs; new drugs (mizoribine *, mycophenolate mofetil, brequinar sodium *) have a more selective effect on immunocompetent cells.
Cyclosporine activates T lymphocytes. Currently, this drug is most common in transplantology and in the treatment of certain autoimmune diseases.
Pharmacokinetics. The bioavailability of cyclosporine when taken orally is 20-50%. Fatty foods reduce bioavailability when the drug is taken in soft gelatin capsules and do not affect the absorption of cyclosporine administered as a microemulsion. Peak concentration is reached 1.3-4 hours after oral administration. Due to its good solubility in fats, cyclosporine is evenly distributed in the body (volume of distribution -
13 l/kg), especially in the liver, lungs, kidneys, pancreas, spleen, fatty tissue, lymph nodes, where the concentration of the drug exceeds the plasma concentration. Cyclosporine does not penetrate well through the blood-brain barrier and into breast milk, although it crosses the placental barrier and is detected in amniotic fluid. 50-60% of the drug accumulates in erythrocytes, 10-20% in leukocytes, the remainder binds to plasma lipoproteins and, to a lesser extent, to albumin. T 1/2 -6 hours. The drug undergoes biotransformation in the liver with the formation of more than 30 metabolites, which are excreted mainly in bile. Elimination is reduced in patients with impaired liver function and in elderly patients.
Pharmacodynamics. Cyclosporine selectively suppresses the activity of CD4 T lymphocytes, inhibits the early phases of the cellular response to antigens and regulatory stimuli by disrupting the function of proteins involved in the activation of T lymphocytes and the expression of genes encoding the synthesis of cytokines (IL-2, IL-3, IL-4, TNF). Cyclosporine also suppresses the chemotaxis of mononuclear phagocytes and the expression of class II antigens of the major histocompatibility complex on the membranes of antigen-presenting cells.
Indications. Cyclosporine remains the main drug for the prevention of graft rejection (during kidney, heart, liver and other organ transplants) as monotherapy or in combination with glucocorticoids. It is also prescribed for autoimmune diseases: Behcet's syndrome, endogenous uveitis, psoriasis, atopic dermatitis, rheumatoid arthritis, Crohn's disease (a type of ulcerative colitis).
NLR. Cyclosporine has a nephrotoxic effect, which often forces discontinuation of the drug. Hypertension, hepatotoxicity, neurotoxicity, hirsutism, gingival hyperplasia, and dyspeptic symptoms develop less frequently.
Interaction with other drugs. Cyclosporine interacts with many drugs by affecting cytochrome P-450. The concentration of cyclosporine is reduced by barbiturates, carbamazepine, rifampicin, sulfonamides, and phenytoin. The concentration of cyclosporine increases with the simultaneous administration of amphotericin B, erythromycin, ketoconazole, glucocorticoids, some calcium antagonists (verapamil, diltiazem), doxycycline. Metoclopramide increases the absorption of cyclosporine.
Azathioprine
Synthetic derivative of 6-mercaptopurine. The immunosuppressive effect of azathioprine is stronger than its cytotoxic effect.
Pharmacokinetics. Bioavailability when taken orally is about 20%. The maximum concentration of the drug is achieved after 1-2 hours. The highest concentrations are created in the tissues of the liver, intestines, as well as in the kidneys, lungs, spleen, and muscles. The drug is quickly biotransformed and has a very variable half-life (about 5 hours on average). Azathioprine and its metabolites are excreted by the kidneys.
Pharmacodynamics. Azathioprine suppresses the proliferation of all rapidly dividing cells, and T-lymphocytes to a greater extent than B-lymphocytes, as a result of disruption of DNA synthesis. Azathioprine has a damaging effect on cells during mitosis, so it is effective both before and after antigen administration.
Indications. During organ transplantation (primarily kidneys) to prevent graft rejection in combination with cyclosporine or glucocorticoids or as monotherapy. Azathioprine is considered a reserve drug for some autoimmune diseases (severe rheumatoid arthritis, refractory to glucocorticoids).
NLR. Bone marrow suppression (leukopenia, thrombocytopenia), gastrointestinal reactions, hepatotoxicity, alopecia, increased susceptibility to infections, mutagenicity, carcinogenicity.
Interaction with other drugs. When administered concomitantly with allopurinol, the toxicity of azathioprine increases. If it is necessary to prescribe these drugs simultaneously, the dose of azathioprine should be reduced by 25-35%.
Cyclophosphamide
Pharmacokinetics. Well absorbed when taken orally, bioavailability is more than 75%. Plasma protein binding is low and is metabolized in the liver. Peak concentration is reached after 2-3 hours. T 1 / 2 3-12 hours. Excreted by the kidneys mainly in the form of metabolites, 5-25% unchanged.
Pharmacodynamics. Cyclophosphamide inhibits DNA synthesis of both proliferating and resting cells and suppresses the activity of B and T lymphocytes. It has a greater effect on B-lymphocytes and, accordingly, on the activity of antibody formation.
Indications. Bone marrow transplantation. In low doses, cyclophosphamide is used in the treatment of autoimmune diseases, such as
such as systemic lupus erythematosus, Wegener's granulomatosis, idiopathic thrombocytopenic purpura, rheumatoid arthritis, dermatomyositis.
NLR. When prescribing large doses, the development of hemorrhagic cystitis, cardiotoxicity, severe pancytopenia, infections, and toxic kidney damage is possible. Anemia and thrombocytopenia develop less frequently. Anaphylactic reactions, hemorrhagic colitis, hepatitis, and stomatitis occur extremely rarely.
Interaction with other drugs. Cyclophosphamide potentiates bone marrow suppression by other myelotoxic drugs. Increased cardiotoxicity is possible when using cyclophosphamide with doxorubicin and increased hepatotoxicity when used simultaneously with azathioprine, chlorambucil, glucocorticoids, and cyclosporine.
Methotrexate
Pharmacokinetics. The maximum concentration in the blood is achieved 1-4 hours after oral administration and 40 minutes after intravenous administration. Bioavailability is 60-70%. T 1/2 -10 hours. Excreted mainly by the kidneys. Part of the drug binds to proteins and can remain in tissues for up to 1 month.
Pharmacodynamics. The use of methotrexate in large doses leads to the suppression of folate-dependent enzymes, purine synthesis and, accordingly, to the death of proliferating cells - a predominantly cytotoxic effect develops.
When prescribed in small and medium doses, the immunosuppressive effect of the drug predominates as a result of suppression of the synthesis of pro-inflammatory cytokines, induction of apoptosis of activated T-lymphocytes, and inhibition of neutrophil motility. Methotrexate also suppresses the humoral component of the immune system and reduces the concentration of immunoglobulins of classes G, M and A.
Indications. Second-line drug for the treatment of rheumatoid arthritis. Prescribed for the treatment of psoriasis refractory to standard therapy, psoriatic arthritis, dermatomyositis.
NLR. Nausea, vomiting, loss of appetite, diarrhea, increased transaminase activity. With long-term use of methotrexate, 40% of patients experience dose-dependent hepatotoxicity with possible development fibrosis and cirrhosis of the liver. Bone marrow suppression, pneumonitis, and anaphylaxis are possible. Has teratogenic and carcinogenic effects.
Interaction with other drugs. Methotrexate increases the hepatotoxicity of other drugs. When combined with phenylbutazone, the risk of bone marrow suppression increases. Penicillins and probenecid * increase, and phenytoin decreases, the concentration of methotrexate in plasma. Parallel administration of pyrimethamine, triamterene, trimethoprim can lead to increased toxic effects of methotrexate.
24.9. CLINICAL PHARMACOLOGY OF MONOCLONAL ANTIBODY PREPARATIONS
Traditional treatment with NSAIDs, glucocorticoids, and classical immunosuppressants is ineffective in 25-50% of patients with rheumatoid arthritis, does not reduce the activity of the disease and does not prevent the progression of osteochondral destruction and disability of patients. The key cytokine in the development of the disease is considered to be tumor necrosis factor-alpha, which is produced by monocytes, macrophages, fibroblasts, and T-B lymphocytes. It causes the development of chronic inflammation, destruction of cartilage and bone, loss of bone mass, acts as a mediator of the inflammatory response and is involved in the modulation of the immune system. TNF-α is important in the development of autoimmune and inflammatory diseases. TNF-α blockers (infliximab, adalimumab) are used as drugs for the treatment of rheumatoid, psoriatic arthritis, ankylosing spondylitis and Crohn's disease.
Infliximab (Remicade*) is a chimeric IgG1 monoclonal antibody consisting of 75% human protein and 25% mouse protein. Immunosuppressive agent, has a high affinity for TNF-α.
The drug quickly binds and forms a stable compound with the soluble and transmembrane forms of human TNF-α, reducing its functional activity. The specificity of infliximab for TNF-α is confirmed by its inability to neutralize the cytotoxic effect of a lymphotoxin cytokine that uses the same receptors as TNF-α.
Pharmacokinetics
Pharmacokinetic parameters (Cmax, volume of distribution, AUC) are dose-dependent. C max after a single intravenous infusion
Zia at a dose of 5 mg/kg is 118 mcg/ml, volume of distribution is 3 l. Final T 1/2 -9.5 days. Displayed within 6 months.
Indications for use
Rheumatoid arthritis (if previous treatment, including methotrexate, is ineffective). Crohn's disease (severe course, with ineffectiveness of standard treatment, including glucocorticoids and/or immunosuppressants).
NLR
Allergic reactions (delayed type): myalgia and/or arthralgia with fever, urticaria, itching, swelling of the face, lips, hands, dysphagia. The use of the drug 2-4 years after the last dose is accompanied by the development of allergic reactions in most patients. NDRs of other organs and systems: dizziness, fainting, “flushes” of blood to the skin of the face, increased or decreased blood pressure, nausea, diarrhea, dyspepsia, anemia, leukopenia, lymphadenopathy.
Interaction with other drugs
Methotrexate reduces the formation of antibodies to infliximab and increases its concentration in plasma.
Adalimumab. By selectively binding to TNF, it blocks its interaction with surface cellular p55 and p75 receptors, neutralizing the functions of TNF. Alters biological responses controlled by TNF, including changes in adhesion molecules that cause leukocyte migration. Reduces the concentration of C-reactive protein, ESR, serum cytokines
(IL-6).
Pharmacokinetics
Absorbed slowly. Bioavailability with a single subcutaneous injection of 40 mg is 64%. TS tah - 5 days. The volume of distribution for intravenous administration is 4.7-6 liters. Concentration in synovial fluid is 31-96% serum. It comes out slowly. Clearance - 12 ml/h; depends on body weight and the presence of antibodies to adalimumab. Clearance and T1/2 do not change significantly at a dose of 0.25-10 mg/kg. Age has a minimal effect on clearance. T 1/2 with intravenous and subcutaneous administration - 2 weeks
(10-20 days).
Indications for use
Exacerbation of moderate and severe rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis.
NLR. Headache, dizziness, paresthesia, increased blood pressure, nausea, abdominal pain, diarrhea, dyspepsia, anemia, lymphopenia. Local reactions: pain, swelling, redness, itching at the injection site.
Contraindications
Hypersensitivity (including to latex), infectious diseases (tuberculosis), age under 18 years, pregnancy, lactation.
Interaction
Single and repeated use with methotrexate reduces the clearance of adalimumab by 29 and 44%, respectively, but this does not require dose adjustment of methotrexate and adalimumab.
Clinical pharmacology and pharmacotherapy: textbook. - 3rd ed., revised. and additional / ed. V. G. Kukesa, A. K. Starodubtseva. - 2012. - 840 p.: ill.