Adrenergic agonists: groups and classification, drugs, mechanism of action and treatment. Treatment of an acute attack of bronchial asthma in the intensive care unit. Principles and methods of treatment of status asthmaticus
7
081. Inhaled, short-acting B2-agonists are the drugs of choice
- a) for maintenance therapy of bronchial asthma
- b) for chronic bronchitis
- c) when relieving attacks of bronchial asthma
082. The following drugs have an M-anticholinergic effect
- a) salbutamol
- b) teopek
- c) berotek
- d) atrovent
083. The disadvantages of M-anticholinergics compared to other bronchodilators are
- a) high frequency of side effects
- b) system effects
- c) later onset of action compared to B2-agonists
085. B-agonists include
- a) disodium chromoglycate
- b) fiupizolid
- c) salbutamol
- d) nedocromil sodium
086. Universal stimulants of the adrenergic system include
- a) atropine
- b) theophylline
- c) adrenaline
087. Selective B2 agonists include
- a) adrenaline
- b) asthmapent
- c) berotek
- d) berodual
088. The most effective way to administer adrenaline to relieve bronchospasm
- a) intravenous drip
- b) inhalation
- c) subcutaneously
089. B2-agonists have the following pharmacological effects
A) bronchodilation, antiallergic effect, activation of the function of the ciliated epithelium, positive chronotropic effect, decreased uterine tone
B) decreased uterine tone, antiallergic effect, suppression of ciliated epithelial function, bronchodilation, negative chronotropic effect
- c) bronchoconstriction, decreased uterine tone, negative chronotropic effect, antiallergic effect
090. The following side effects are most typical for B2-agonists
- a) cardiac stimulation
- b) toxic effect on the myocardium
- c) ventricular fibrillation
- d) rebound syndrome
- e) dilation of blood vessels in the submucosal layer of the bronchi
- e) hypokalemia
- g) all of the above
091. Side effects when using theophylline preparations are as follows
- a) nausea, vomiting, headache, bradycardia, rhythm disturbance
- b) dry mouth, nausea, headache, arrhythmia, bradycardia
- c) tremor, nausea, headache, rhythm disturbance
092. The effect of theophylline on the cerebral cortex
- a) increases excitability
- b) reduces excitability
- c) does not work
093. Theophylline acts on the respiratory center, causing
- a) excitement
- b) oppression
- c) does not work
094. Coronary blood flow under the influence of theophylline
- a) increases
- b) decreases
- c) does not change
095. Myocardial contractility when using theophylline
- a) does not change
- b) increases
- c) decreases
096. The most effective way of administering aminophylline during an acute attack of asthma is
- a) intramuscular
- b) rectal (suppository, microenema)
- c) inhalation
- d) intravenous
- e) oral
097. To prevent an attack of bronchial asthma, the most effective is the use of aminophylline
- a) inside
- b) intravenously
- c) intramuscularly
- d) rectally (suppository, microenema)
- d) inhalation
098. The effect of ascorbic acid on aminophylline solutions with simultaneous intravenous administration
- a) enhances
- b) destroys aminophylline
- c) has no effect
099. Removal of aminophylline from the body in patients with severe manifestations of respiratory failure
- a) slow
- b) accelerated
- c) not changed
100. Mechanism of action of theophylline
- a) stimulation of B receptors
- b) inhibition of phosphodiesel
- c) inhibition of phospholipase A2
101. Serum theophylline concentration causing bronchodilation
- a) 5.5 µg/ml
- b) 10 µg/ml
- c) 20 µg/ml
- d) over 20 µg/ml
102. Concentration of theophylline in blood serum causing toxic manifestations
- a) 5.5 µg/ml
- b) 10 µg/ml
- c) 20 µg/ml
- d) over 20 µg/ml
103. Indications for inhalation of sodium cromoglycate
- a) treatment of an asthmatic attack
- b) treatment of an acute attack of bronchial asthma
- c) prevention of an attack of bronchial asthma
104. Disodium chromoglycate
- a) effective for status asthmaticus
- b) effective in some cases of non-allergic bronchial asthma
- c) is a drug, such as a steroid
- d) used only in short courses
105. Ditek is used for bronchial asthma
- a) to relieve an acute attack of suffocation
- b) to prevent an acute attack
- c) for the prevention and relief of suffocation
- d) for the treatment of status asthmaticus
106. Rarely used drugs in the treatment of bronchial asthma include
- a) anticholinergics
- b) mucolytics
- c) 1st generation antihistamines
- d) theophylline
107. Exercise-induced asthma can be prevented by prophylactic use
- a) beclomethasone dipropionase
- b) ipratropium bromide
- c) B2-agonists
- d) Troventol
108. Spirotent is
- a) long-acting oral B-agonist
- b) M-cholinergic receptor blocker
- c) contact laxative
- d) inhaled anti-inflammatory drug
109. Oral B2-agonists are the drug of choice
- a) with bronchial asthma with predominant development of attacks at night
- b) with chronic obstructive bronchitis
- c) with pollen bronchial asthma
- d) with all of the above
110. Berodual refers
- a) to combined bronchodilators
- b) to non-selective B-agonists
- c) to inhaled anti-inflammatory drugs
111. Berodural is the drug of choice
- a) for chronic asthmatic bronchitis
- b) for bronchial asthma in elderly patients
- c) with episodic attacks of bronchial asthma at a young age
- d) with a combination of chronic obstructive bronchitis and bronchial asthma
112. The advantages of Berodual over other bronchodilators are all listed below, except
- a) the rapid onset of the effect of berodual in combination with its long-term action
- b) Berodual does not have a cholinolytic effect on the bronchi
- c) berodual is effective for both asthma and bronchitis
- d) high efficiency of berodual combined with a low incidence of side effects
113. Inhaled glucocorticosteroids are used
- a) in acute pneumonia
- b) with atopic bronchial asthma
- c) with systemic lupus erythematosus
- d) for chronic bronchitis
114. For atopic bronchial asthma, inhaled glucocorticosteroids are used in the case of (2)
- a) mild course
- b) middle current
- c) severe course
- d) ineffectiveness of sodium cromoglycate within 4 weeks
- e) ineffectiveness of sodium cromoglycate within 2 weeks
115. The indication for reducing the dose of inhaled glucocorticosteroids is
- a) status asthmaticus
- b) transition of the disease into remission
- c) increasing the need for B-agonists over 3-4 inhalations per day
- d) increasing the need for B-agonists over 8 inhalations per day
116. Inhaled glucocorticosteroids include everything except (2)
- a) becotida
- b) beklomet
- c) bricanila
- d) pulmonary cortex
- e) kenalog
- e) Ingacorta
117. Systemic side effects of flunisolide, budesonide
- a) Itsenko-Cushing syndrome
- b) ulcerogenic effect
- c) steroid diabetes
- d) osteoporosis
- d) extremely rare
118. Advantage of flunisolide over beclomethasone
- a) higher efficiency
- b) a smaller dose of corticosteroid in one inhalation
119. The mechanism of anti-inflammatory activity of GC, except
- a) restoration of tissue response to catecholamines
- b) decrease in antiphosphodiesterase activity
- c) decrease in ATPase level
- d) reducing the level of guanylate cyclase activity
- e) stimulation of the production of phospholipase A2 inhibitors
120. Aerosols of short-acting B2-agonists no more than 3-4 times a day are used for bronchial asthma
- a) moderate severity (2-3rd degree)
- b) severe (4th degree)
121. Long-acting B2-agonists include
- a) bricanil
- b) ventolin
- c) salmeterol
- d) orciprenaline
- e) formoterol
001. The most common primary pathogens of acute bronchitis are:
- a) bacteria
- b) mycoplasma,
- c) viruses
002. Acute bronchitis with a prolonged course is considered to be a disease lasting:
- a) more than 2 weeks,
- b) more than 1 month,
- c) more than 2 months
003. The leading initial complaint of patients with acute bronchitis is:
- a) cough with sputum,
- b) dry cough,
- c) chest pain
004. Shortness of breath is most typical:
- a) for proximal acute bronchitis,
- b) for distal acute bronchitis,
- c) for acute bronchiolitis
005. The leading clinical sign of bronchiolitis is:
- a) cough,
- b) chest pain,
- c) shortness of breath
006. When auscultating the lungs in patients with acute bronchitis, the most common occurrence is:
- a) pleural friction noise,
- b) moist rales,
- c) dry wheezing,
- d) crepitation
007. Bronchial obstruction syndrome is typical (2)
- a) for distal acute bronchitis,
- b) for proximal acute bronchitis,
- c) for acute bronchiolitis
008. The leading auscultatory symptoms of acute bronchiolitis are: (2)
- a) fine bubbling moist rales,
- b) large bubble moist rales,
- c) pleural friction noise,
- d) weakened vesicular respiration,
- d) crepitus,
- e) hard breathing
009. Indications for prescribing antibacterial therapy in patients with acute bronchitis are:
- a) the appearance of purulent sputum,
- b) addition of bronchopneumonia,
- c) exacerbation of chronic foci of infection,
- d) weakened condition of patients,
- e) the presence of severe chronic concomitant pathology
- e) all of the above are true.
010. The triad of symptoms most characteristic of chronic bronchitis include: (3)
- a) cyanosis,
- b) secretion of sputum,
- c) cough,
- d) pulmonary hypertension,
- e) chest pain,
- e) shortness of breath,
- g) low-grade fever
011. Moist wheezing in chronic bronchitis:
- a) do not meet,
- b) are a sign of bronchial hypersecretion,
- c) indicate the presence of diffuse peribronchial sclerosis
012. The main method for diagnosing tracheobronchial dyskinesia is:
- a) spirography,
- b) chest x-ray,
- c) fibrobronchoscopy,
- d) bronchography
013. The WHO criterion for chronic bronchitis is the duration of cough:
- a) at least 6 months a year for 2 years in a row,
- b) more than 4 months in a given year,
- c) at least 3 months a year for 2 years in a row,
- d) at least 2 months a year for 3 years in a row
014. Cough is more pronounced:
- a) with distal bronchitis,
- b) with proximal bronchitis
015. Chest radiography for chronic obstructive bronchitis is characterized by: (3)
- a) local pneumofibrosis,
- b) diffuse pneumofibrosis mainly in the lower parts,
- c) diffuse pneumofibrosis mainly in the upper parts,
- d) thickening of the walls of the bronchi,
- e) pleural adhesions,
- f) signs of pulmonary emphysema
The article describes the current state of the problem of bronchial asthma. In the United States, 14 billion dollars are spent annually on the treatment of bronchial asthma, a quarter of which is due to its exacerbation (hospitalization, visits to the emergency room). Criteria for exacerbation and hospitalization are multifactorial in nature and may vary slightly in different centers (results of one spirometry or spirometry and clinical signs of the disease, etc.)
Drug treatment
Short-acting B2-agonists
Short-acting B2 agonists are the most commonly used. Methods of drug delivery (nebulizer versus metered-dose inhaler with retention chamber) are discussed and their effectiveness is compared.
Using either of them showed the same results. However, the use of a metered dose inhaler with a chamber (or spacer) is associated with fewer side effects (tachycardia, tremor), especially in children. The economic advantage is also on the side of the metered dose inhaler with a chamber.
In cases of severe exacerbation of asthma (status asthmaticus), the need for prolonged inhalation of a B2 agonist makes the use of a nebulizer more convenient and effective.
Attempts to determine the optimal dose and interval of use of B2-agonists have led nowhere. It was found that small doses are equivalent in effectiveness to maximum doses (up to 5-10 mg per nebulizer dose). The clinical effect of bronchodilation plateaus, so additional doses of B2 agonists cause only side effects. There are some recommendations for titrating the B2 agonist to a plateau effect, guided by pulmonary function tests.
Anticholinergic bronchodilators
The use of ipratropium bromide, as an addition to B2-agonists, in cases of moderate asthma has shown an improvement in pulmonary tests (FEV1 and PEF) and a decrease in the frequency of hospitalizations with exacerbation of bronchial asthma. In case of exacerbation of bronchial asthma, the effectiveness of ipratropium bromide has also been proven (however, the “dose-effect” issue has not been studied precisely).
Systemic corticosteroids
Many guidelines recommend corticosteroids as first-line drugs for exacerbation of asthma; an analysis of 12 randomized clinical trials found a significant reduction in hospitalizations when taking systemic corticosteroids. Intravenous administration of corticosteroids is recommended for patients with severe exacerbation of bronchial asthma (intubated patients, patients with vomiting, impaired consciousness, impaired swallowing, etc.).
Magnesium sulfate
For exacerbation of bronchial asthma in adults (the pediatric dose is not written), the drug is administered in an amount of 2 g, over 20 minutes, intravenously. Magnesium sulfate is a safe and effective drug in addition to b2-agonists and corticosteroids. Numerous studies have proven the effectiveness of early administration of magnesium sulfate for exacerbation of bronchial asthma in adults and children.
Inhaled corticosteroids
Used for basic treatment of bronchial asthma without exacerbation. However, there is also some evidence of the effectiveness of inhaled corticosteroids in exacerbation of bronchial asthma due to their vasoconstrictor properties prior to their anti-inflammatory effects.
Additional medicines
Intramuscular administration of adrenaline is recommended if there is evidence of the anaphylactic nature of exacerbation of bronchial asthma. Long-acting B2-agonists and leukotriene receptor antagonists are recommended as an adjunct to the basic treatment of poorly controlled asthma.
Respiratory support
Non-invasive ventilation
There is little evidence base for the effectiveness of non-invasive ventilation for exacerbation of bronchial asthma. The main idea is to avoid intubation. Full or partial face masks are used and positive pressure ventilation is used (biphasic respiratory support, BiPAP, for example).
Invasive mechanical ventilation (tracheal intubation)
When everything turns out to be ineffective, tracheal intubation is performed. Cases of invasive mechanical ventilation for bronchial asthma are quite rare, and accordingly the research base is scarce. As a rule, mechanical ventilation in such patients may be accompanied by barotrauma and other complications. Ventilation modes, use of heliox, etc. are not covered in this article. Not a single word has been written about our beloved aminophylline...
Each drug belongs to a specific pharmacological group. This means that some medications have the same mechanism of action, indications for use and side effects. One of the large pharmacological groups is beta-adrenergic agonists. These drugs are widely used in the treatment of respiratory and cardiovascular pathologies.
What are B-adrenergic agonists?
Beta-agonists are a group of medications that are used in the treatment of various diseases. In the body, they bind to specific receptors located in the smooth muscles of the bronchi, uterus, heart, and vascular tissue. This interaction causes stimulation of beta cells. As a result, various physiological processes are activated. When B-adrenergic agonists bind to receptors, the production of biological substances such as dopamine and adrenaline is stimulated. Another name for these compounds is beta-agonists. Their main effects are an increase in heart rate, an increase in blood pressure and an improvement in bronchial conduction.

Beta-agonists: action in the body
Beta agonists are divided into B1 and B2 adrenergic agonists. Receptors for these substances are located in internal organs. When bound to them, beta-agonists lead to the activation of many processes in the body. The following effects of B-agonists are distinguished:
- Increased cardiac automaticity and improved conduction.
- Increased heart rate.
- Acceleration of lipolysis. When using B1-adrenergic agonists, free fatty acids appear in the blood, which are products of the breakdown of triglycerides.
- Increased blood pressure. This action is due to stimulation of the renin-angiotensin-aldosterone system (RAAS).
The listed changes in the body are caused by the binding of adrenergic agonists to B1 receptors. They are located in the heart muscle, blood vessels, adipose tissue and kidney cells.

B2 receptors are located in the bronchi, uterus, skeletal muscles, and central nervous system. In addition, they are present in the heart and blood vessels. Beta-2 adrenergic agonists cause the following effects:
- Improvement of bronchial conductivity. This action is due to the relaxation of smooth muscles.
- Acceleration of glycogenolysis in muscles. As a result, skeletal muscles contract faster and stronger.
- Relaxation of the myometrium.
- Acceleration of glycogenolysis in liver cells. This leads to increased blood sugar levels.
- Increased heart rate.
What drugs belong to the group of B-adrenergic agonists?
Doctors often prescribe beta-agonists. Drugs belonging to this pharmacological group are divided into short- and fast-acting medications. In addition, there are drugs that have a selective effect only on certain organs. Some drugs act directly on B1 and B2 receptors. The most well-known medications from the group of beta-adrenergic agonists are the drugs “Salbutamol”, “Fenoterol”, “Dopamine”. β-agonists are used in the treatment of pulmonary and cardiac diseases. Also, some of them are used in the intensive care unit (the drug “Dobutamine”). Less commonly, drugs of this group are used in gynecological practice.

Classification of beta-agonists: types of medications
Beta-agonists are a pharmacological group that includes a large number of medications. Therefore, they are divided into several groups. Classification of B-agonists includes:
- Non-selective beta-agonists. This group includes the medications “Orciprenaline” and “Isoprenaline”.
- Selective B1-adrenergic agonists. They are used in cardiology and intensive care units. Representatives of this group are the drugs “Dobutamine” and “Dopamine”.
- Selective beta-2 adrenergic agonists. This group includes medications used for diseases of the respiratory system. In turn, selective B2 agonists are divided into short-acting drugs and drugs with a long-term effect. The first group includes the medications Fenoterol, Terbutaline, Salbutamol and Hexoprenaline. Long-acting drugs are the drugs Formoterol, Salmeterol and Indacaterol.
Indications for the use of B-adrenergic agonists
Indications for the use of B-adrenergic agonists depend on the type of drug. Non-selective beta-agonists are currently practically not used. Previously, they were used to treat certain types of rhythm disturbances, deterioration of cardiac conduction, and bronchial asthma. Now doctors prefer to prescribe selective B-agonists. Their advantage is that they have far fewer side effects. In addition, selective drugs are more convenient to use, since they affect only certain organs.
Indications for the use of B1-adrenergic agonists:
- Shock of any etiology.
- Collapse.
- Decompensated heart defects.
- Rarely - severe ischemic heart disease.

B2-agonists are prescribed for bronchial asthma and chronic obstructive pulmonary disease. In most cases, these drugs are used in the form of aerosols. Sometimes the drug Fenoterol is used in gynecological practice to slow down labor and prevent miscarriage. In this case, the drug is administered intravenously.
In what cases are B-agonists contraindicated?

B2-agonists are contraindicated in the following cases:
- Intolerance to beta-agonists.
- Pregnancy complicated by bleeding, placental abruption, or threatened miscarriage.
- Children under 2 years of age.
- Inflammatory processes in the myocardium, rhythm disturbances.
- Diabetes.
- Aortic stenosis.
- Arterial hypertension.
- Acute heart failure.
- Thyrotoxicosis.
Medication "Salbutamol": instructions for use
The drug "Salbutamol" is a short-acting B2 agonist. It is used for bronchial obstruction syndrome. Most often used in aerosols of 1-2 doses (0.1-0.2 mg). For children, it is preferable to inhale through a nebulizer. There is also a tablet form of the drug. The dosage for adults is 6-16 mg per day.
"Salbutamol": price of the drug
The medication is used as monotherapy for mild bronchial asthma. If the patient has a moderate or severe stage of the disease, long-acting medications (long-acting beta-agonists) are used. They are the basic therapy for bronchial asthma. To quickly relieve an attack of suffocation, the drug “Salbutamol” is used. The price of the medicine ranges from 50 to 160 rubles, depending on the manufacturer and the dose contained in the bottle.
The mechanism of action is associated with stimulation of beta-2 adrenergic receptors and relaxation of the smooth muscles of large and small bronchi. They improve mucociliary clearance, reduce vascular permeability and plasma exudation, stabilize the mast cell membrane and reduce the release of mediators.
Main drugs:
Short fast action(release form - metered dose aerosol inhaler, solutions for nebulizers): salbutamol, fenoterol (Berotec). The onset of action is in 1-3 minutes, the duration of action is 4-6 hours.
Long-lasting fast action(release form - powder inhaler): formoterol (oxis turbuhaler). The onset of action is after 1-3 minutes, the duration of action is at least 12 hours.
Long lasting slow action: salmeterol (Serevent). Release form: metered dose aerosol inhaler (MDI). The onset of action is after 15-20 minutes, duration is at least 12 hours.
Side effects.
Cardiovascular system: sinus tachycardia, arrhythmias, hypotension, QT prolongation.
Respiratory system: hypoxemia, paradoxical bronchospasm.
Nervous system: tremor, dizziness, insomnia.
Gastrointestinal tract: nausea, vomiting.
Metabolic: hypokalemia, hyperglycemia, hyperinsulinemia.
2. Anticholinergic drugs.
The main mechanism of action is bronchodilation, which is caused by the blockade of muscarinic cholinergic receptors, as a result of which the reflex narrowing of the bronchi caused by irritation of cholinergic receptors is suppressed and the tone of the vagus nerve is weakened. In elderly patients with concomitant cardiovascular pathology, they are used as an alternative to beta-2 agonists.
Basic drugs.
Short acting: ipratropium bromide (Atrovent). Release form: MDI, solution for nebulizers. The onset of action is 5-30 minutes, duration is 4-8 hours.
Long-lasting: Tiotropium bromide (Spiriva). Release form: powder inhaler. The onset of action is 30-60 minutes, duration is 24 hours or more.
Tachyphylaxis does not develop, and sensitivity to the drug does not decrease.
Side effects.
Local: dry mouth, cough, pharyngitis, bitter taste, nausea.
There may be an exacerbation of glaucoma when used through a nebulizer.
Systemic (rare): tachycardia, urinary retention, constipation.
Devices for delivering inhaled drugs (Table 7):
Metered-dose aerosol inhalers (-/+ spacer)
Powder inhalers
Nebulizers
Rice. 2. Spacer.
1 - mouthpiece, 2 - inhaler, 3 - hole for inhaler, 4 - spacer body.
1. Ultrasonic, using the energy of a piezoelectric crystal;
2. Jet (compressor), air stream energy:
2.1. Nebulizers synchronized with breathing
2.2. Breath-activated nebulizers
2.3. Convection nebulizers
The method of delivering inhaled drugs to the lungs is metered dose inhalers with or without spacers (Fig. 2) and powder inhalers (Fig. 3).

Rice. 3. The structure of a powder inhaler - turbuhaler.
Recently, a modern delivery method has appeared - a nebulizer (Fig. 4). Advantages of nebulizer therapy: the ability to deliver a large dose of the drug, ease of use (no need to coordinate inhalation and release of the drug), can be used in severe conditions and at an early age.

Rice. 4. Scheme of a jet nebulizer.
For quotation: Sinopalnikov A.I., Klyachkina I.L. b2-agonists: role and place in the treatment of bronchial asthma // Breast Cancer. 2002. No. 5. P. 236
State Institute for Advanced Training of Physicians of the Ministry of Defense of the Russian Federation, Moscow
Introduction Therapy of bronchial asthma (BA) can be divided into two main areas. The first is symptomatic therapy, which quickly and effectively relieves bronchospasm, the leading clinical symptom of asthma. The second is anti-inflammatory therapy, which helps to modify the main pathogenetic mechanism of the disease, namely, inflammation of the mucous membrane of the respiratory tract (RT).
The central place among the means of symptomatic control of asthma is obviously occupied by b2-agonists, characterized by pronounced bronchodilator activity (and bronchoprotective effect) and a minimal number of undesirable side effects when used correctly.
Brief history b 2 -agonists The history of the use of b -agonists in the 20th century is the consistent development and introduction into clinical practice of drugs with ever increasing b 2 -adrenergic selectivity and increasing duration of action.
First time sympathomimetic adrenalin (epinephrine) was used in the treatment of patients with asthma in 1900. At first, adrenaline was widely used both in injection form and in the form of inhalations. However, doctors' dissatisfaction with the short duration of action (1-1.5 hours) and the large number of negative side effects of the drug stimulated the further search for more “attractive” drugs.
In 1940 appeared isoproterenol - synthetic catecholamine. It was destroyed in the liver as quickly as adrenaline (with the participation of the enzyme catechol-o-methyltransferase - COMT), and therefore was characterized by a short duration of action (1-1.5 hours), and the metabolites formed as a result of the biotransformation of isoproterenol (methoxyprenaline) had b-adrenergic blocking effect. At the same time, isoproterenol was free from such undesirable effects inherent in adrenaline as headache, urinary retention, arterial hypertension, etc. The study of the pharmacological properties of isoproterenol led to the establishment of heterogeneity of adrenergic receptors. In relation to the latter, adrenaline turned out to be a universal direct a-b-agonist, and isoproterenol was the first short-acting non-selective b-agonist.
The first selective b 2 -agonist appeared in 1970. salbutamol , characterized by minimal and clinically insignificant activity against a - and b 1 receptors. It has rightfully acquired the status of the “gold standard” in the series of b 2 agonists. Salbutamol was followed by the introduction into clinical practice of other b 2 -agonists (terbutaline, fenoterol, etc.). These drugs turned out to be as effective as bronchodilators as non-selective β-agonists, since the bronchodilator effect of sympathomimetics is realized only through β 2 -adrenergic receptors. At the same time, b 2 -agonists demonstrate a significantly less pronounced stimulating effect on the heart (batmotropic, dromotropic, chronotropic) compared to the b 1 -b 2 -agonist isoproterenol.
Some differences in b 2 -agonist selectivity are not of major clinical significance. The higher incidence of adverse cardiovascular effects when taking fenoterol (compared to salbutamol and terbutaline) can be explained by the larger effective dose of the drug and, in part, faster systemic absorption. The new drugs retained their speed of action (the onset of effect in the first 3-5 minutes after inhalation), characteristic of all previous b-agonists, with a noticeable increase in the duration of their action to 4-6 hours (less pronounced in severe BA). This improved the ability to control asthma symptoms during the day, but “did not save” from night attacks.
The emerging possibility of taking individual b 2 -agonists orally (salbutamol, terbutaline, formoterol, bambuterol) to some extent solved the problem of controlling nocturnal asthma attacks. However, the need to take significantly higher doses (almost 20 times more than for inhalation use) contributed to the emergence of adverse events associated with stimulation of a - and b 1 -adrenergic receptors. In addition, lower therapeutic efficacy of these drugs was also revealed.
The advent of long-acting inhaled b2-agonists - salmeterol and formoterol - has significantly changed the possibilities of asthma therapy. First to appear on the market salmeterol - a highly selective b 2 -agonist, demonstrating a duration of action of at least 12 hours, but with a slow onset of action. Soon he was “joined” formoterol , also a highly selective b 2 -agonist with a 12-hour action, but with a rate of development of bronchodilation effect similar to that of salbutamol. Already in the first years of use of long-acting b 2 -agonists, it was noted that they help reduce exacerbations of asthma, reduce the number of hospitalizations, and also reduce the need for inhaled corticosteroids (ICS).
The most effective route of administering medications for asthma, including b 2 -agonists, is inhalation. Important advantages of this route are the possibility of direct delivery of drugs to the target organ (which largely ensures the speed of action of bronchodilators) and minimization of undesirable effects. Of the currently known delivery vehicles, the most commonly used are metered dose aerosol inhalers (MDIs), less commonly metered dose powder inhalers (MDIs) and nebulizers. Oral b 2 -agonists in the form of tablets or syrups are used extremely rarely, mainly as an additional remedy for frequent nocturnal asthma symptoms or a high need for inhaled short-acting b 2 -agonists in patients receiving high doses of ICS (equivalent to 1000 mcg of beclomethasone per day or more ) .
Mechanisms of action b 2 -agonists b 2 -agonists cause bronchodilation primarily as a result of direct stimulation of b 2 -adrenergic receptors in the smooth muscles of the respiratory tract. Evidence for this mechanism has been obtained as in vitro(when exposed to isoproterenol, relaxation of the human bronchi and segments of lung tissue occurred), and in vivo(rapid drop in DP resistance after inhalation of a bronchodilator).
Stimulation of b-adrenergic receptors leads to activation of adenylate cyclase, which forms a complex with G-protein (Fig. 1), under the influence of which the content of intracellular cyclic adenosine-3,5-monophosphate (cAMP) increases. The latter leads to the activation of a specific kinase (protein kinase A), which phosphorylates some intracellular proteins, resulting in a decrease in the intracellular calcium concentration (its active “pumping” from the cell to the extracellular space), phosphoinositide hydrolysis is inhibited, myosin light chain kinases are inhibited and, finally, , large calcium-activated potassium channels “open”, causing repolarization (relaxation) of smooth muscle cells and sequestration of calcium into the extracellular depot. It must be said that b 2 -agonists can bind to potassium channels and directly cause relaxation of smooth muscle cells, regardless of an increase in intracellular cAMP concentration.
Fig.1. Molecular mechanisms involved in the bronchodilation effect of b2-agonists (explanations in the text). K Ca - large calcium-activated potassium channel; ATP - adenosine triphosphate; cAMP - cyclic adenosine-3,5-monosphate
b 2 -agonists are considered as functional antagonists that cause the reverse development of bronchoconstriction, regardless of the constrictor effect that has taken place. This circumstance seems extremely important, since many mediators (inflammatory mediators and neurotransmitters) have a bronchoconstrictor effect.As a result of the effect on b -adrenergic receptors localized in various parts of the DP (Table 1), additional effects of b 2 -agonists are revealed, which explain the possibility of prophylactic use of drugs. These include inhibition of the release of mediators from inflammatory cells, reduction of capillary permeability (preventing the development of edema of the bronchial mucosa), inhibition of cholinergic transmission (reduction of cholinergic reflex bronchoconstriction), modulation of mucus production by submucosal glands and, therefore, optimization of mucociliary clearance (Fig. 2).
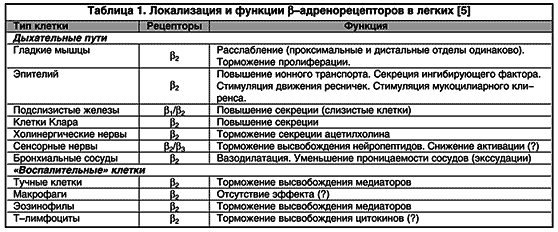

Rice. 2. Direct and indirect bronchodilatory effect of b 2 -agonists (explanations in the text). E - eosinophil; MC - mast cell; CN - cholinergic nerve; SMC - smooth muscle cell
According to the microkinetic diffusion theory of G. Andersen, the duration and time of onset of action of b 2 -agonists are related to their physicochemical properties (primarily the lipophilicity/hydrophilicity of the molecule) and the features of the mechanism of action. Salbutamol - hydrophilic compound. Once in the aqueous environment of the extracellular space, it quickly penetrates into the “core” of the receptor and, after the connection with it ceases, is removed by diffusion (Fig. 3). Salmeterol , created on the basis of salbutamol, a highly lipophilic drug, quickly penetrates the membranes of respiratory tract cells that act as a depot, and then slowly diffuses through the receptor membrane, causing its prolonged activation and a later onset of action. Lipophilicity formoterol less than that of salmeterol, therefore it forms a depot in the plasma membrane, from where it diffuses into the extracellular environment and then simultaneously binds to the b-adrenergic receptor and lipids, which determines both the speed of onset of the effect and an increase in its duration (Fig. 3). The long-lasting effect of salmeterol and formoterol is explained by their ability to remain for a long time in the bilayer of cell membranes of smooth muscle cells in close proximity to b 2 -adrenergic receptors and interact with the latter.

Rice. 3. Mechanism of action of b 2 -agonists (explanations in the text)
When researching in vitro a spasmodic muscle relaxes more quickly when formoterol is added than with salmeterol. This confirms that salmeterol is a partial b 2 receptor agonist relative to formoterol.
Racemates Selective b 2 -agonists are racemic mixtures (50:50) of two optical isomers - R and S. It has been established that the pharmacological activity of R-isomers is 20-100 times higher than S-isomers. The R-isomer of salbutamol has been shown to exhibit bronchodilator properties. At the same time, the S-isomer exhibits exactly the opposite properties: a pro-inflammatory effect, an increase in hyperreactivity of the respiratory tract, increased bronchospasm, in addition, it is metabolized much more slowly. Recently, a new drug was created containing only the R-isomer ( levalbuterol
). It exists so far only in a solution for nebulizers and has a better therapeutic effect than racemic salbutamol, since levalbuterol demonstrates an equivalent effect at a dose equal to 25% of the racemic mixture (there is no opposing S-isomer, and the number of adverse events is reduced).
Selectivity b 2 -agonists The goal of using selective b 2 -agonists is to provide bronchodilation while avoiding the side effects induced by stimulation of the a and b 1 receptors. In most cases, moderate use of b 2 -agonists does not lead to the development of undesirable effects. However, selectivity cannot completely eliminate the risk of their development, and there are several explanations for this.
First of all, selectivity to b 2 -adrenergic receptors is always relative and dose-dependent. Minor activation of a - and b 1 -adrenergic receptors, imperceptible at usual average therapeutic doses, becomes clinically significant when the dose of the drug or the frequency of its administration during the day is increased. The dose-dependent effect of b 2 -agonists must be taken into account when treating exacerbations of asthma, especially life-threatening conditions, when repeated inhalations for a short time (several hours) are 5-10 times higher than the permissible daily dose.
b 2 receptors are widely represented in the DP (Table 1). Their density increases as the diameter of the bronchi decreases, and in patients with asthma, the density of b 2 receptors in the respiratory tract is higher than in healthy people. Numerous b 2 -adrenergic receptors are found on the surface of mast cells, neutrophils, eosinophils, and lymphocytes. And at the same time, b 2 receptors are found in a variety of tissues and organs, especially in the left ventricle, where they make up 14% of all b -adrenergic receptors, and in the right atrium - 26% of all b -adrenergic receptors. Stimulation of these receptors can lead to adverse events including tachycardia, atrial flutter, and myocardial ischemia. Stimulation of b 2 receptors in skeletal muscles can cause muscle tremors. Activation of large potassium channels can contribute to the development of hypokalemia and, as a consequence, prolongation of the QT interval and heart rhythm disturbances, incl. fatal. With systemic administration of large doses of drugs, metabolic effects may be observed (increased levels of free fatty acids in the blood serum, insulin, glucose, pyruvate and lactate).
When vascular b 2 receptors are stimulated, vasodilation develops and a possible decrease in diastolic blood pressure. Undesirable cardiac effects are especially pronounced in conditions of severe hypoxia during exacerbations of asthma - an increase in venous return (especially in the orthopneic position) can cause the development of Bezold-Jarisch syndrome with subsequent cardiac arrest.
Connection between b 2 -agonists and inflammation in the DP In connection with the widespread use of short-acting b 2 -agonists, as well as the introduction of long-acting inhaled b 2 -agonists into clinical practice, the question of whether these drugs have an anti-inflammatory effect has become particularly relevant. Undoubtedly, the anti-inflammatory effect of b2-agonists, which helps modify acute bronchial inflammation, can be considered to be inhibition of the release of inflammatory mediators from mast cells and a decrease in capillary permeability. At the same time, during a biopsy of the bronchial mucosa of patients with asthma who regularly took b2-agonists, it was found that the number of inflammatory cells, incl. and activated (macrophages, eosinophils, lymphocytes) does not decrease.
Moreover, theoretically, regular use of b 2 -agonists can even lead to aggravation of inflammation in the DP. Thus, b2-agonist-induced bronchodilation allows for deeper inhalation, which can result in greater allergen exposure.
In addition, regular use of b2-agonists may mask a developing exacerbation, thereby delaying the initiation or intensification of true anti-inflammatory therapy.
Potential risks of using b 2 -agonists Tolerance Frequent, regular use of inhaled b 2 -agonists may lead to the development of tolerance (desensitization) to them. The accumulation of cAMP promotes the transition of the receptor to an inactive state. Excessively intense stimulation of b-adrenergic receptors contributes to the development of desensitization (a decrease in receptor sensitivity as a result of uncoupling of the receptor from G-protein and adenylate cyclase). When excessive stimulation persists, the number of receptors on the cell surface decreases (“down” regulation). It should be noted that b-receptors of smooth muscles of the respiratory tract have a fairly significant reserve and therefore they are more resistant to desensitization than receptors of non-respiratory zones (for example, skeletal muscles or those regulating metabolism). It has been established that healthy individuals quickly develop tolerance to high doses of salbutamol, but not to fenoterol and terbutaline. At the same time, in patients with asthma, tolerance to the bronchodilator effect of b2-agonists rarely appears; tolerance to their bronchoprotective effect develops much more often.
A decrease in the bronchoprotective effect of b 2 -agonists with their regular, frequent use equally applies to both short-acting and long-acting drugs, even against the background of basic therapy with inhaled corticosteroids. At the same time, we are not talking about a complete loss of bronchoprotection, but about a slight decrease in its initial level. H. J. van der Woude et al. found that against the background of regular use of formoterol and salmeterol by patients with asthma, the bronchodilator effect of the latter does not decrease; the bronchoprotective effect is higher for formoterol, but the bronchodilator effect of salbutamol is much less pronounced.
Desensitization develops over a long period of time, over several days or weeks, in contrast to tachyphylaxis, which develops very quickly and is not associated with the functional state of the receptors. This circumstance explains the decrease in the effectiveness of treatment and requires limiting the frequency of use of b 2 -agonists.
Many researchers associate individual variability in the response to b2-agonists and the development of tolerance to their bronchodilator effect with genetic polymorphism of genes. The b 2 -adrenergic receptor gene is localized on chromosome 5q. A significant impact on the course of asthma and the effectiveness of treatment is exerted by changes in the amino acid sequence of b 2 -adrenergic receptors, in particular, the movement of amino acids in codons 16 and 27. The influence of gene polymorphism does not extend to the variability of the bronchoprotective effect. To be fair, it should be noted that these data are not confirmed in all works.
b 2 -agonists and mortality of patients with asthma
Serious doubts about the safety of inhaled b-agonists arose in the 60s of the twentieth century, when an “epidemic of deaths” among patients with asthma broke out in a number of countries, including England, Australia, and New Zealand. At the same time, it has been suggested that there is a connection between sympathomimetic therapy and an increase in mortality from asthma. The cause-and-effect relationship between the use of b-agonists (isoproterenol) and increased mortality was not established at that time, and based on the results of retrospective studies it was almost impossible to prove them. The link between fenoterol use and increased mortality from asthma in New Zealand in the 1980s was proven, as it was found that this drug was more often prescribed in cases of fatal asthma, compared with well-controlled disease. This connection was indirectly confirmed by a decrease in mortality, which coincided with the abolition of widespread use of fenoterol (with a general increase in sales of other b 2 -agonists). In this regard, the results of an epidemiological study in Canada, which aimed to study the possible relationship between the frequency of deaths and prescribed medications, are indicative. An increased incidence of death has been shown to be associated with high-dose therapy with any of the available inhaled b 2 -agonists. The risk of fatal outcome was greatest with fenoterol, but mortality rates were not significantly different when compared with equivalent doses of salbutamol.
At the same time, the connection between high-dose b 2 -agonist therapy and an increase in mortality from asthma cannot be reliably proven, since patients with more severe and poorly controlled asthma more often resort to high doses of b 2 -agonists and, conversely, less often to effective anti-inflammatory drugs. In addition, high doses of b2-agonists mask the signs of an increasingly fatal exacerbation of asthma.
Dosage regimen Inhaled short-acting b 2 -agonists There is no doubt that inhaled short-acting b2-agonists are the drugs of choice for situational symptomatic control of asthma, as well as for preventing the development of symptoms of exercise asthma (EA). Regular use of inhaled beta-agonists may lead to loss of adequate control over the course of the disease. Thus, in a study conducted by M.R. Sears et al. in New Zealand, studied bronchial hyperresponsiveness, morning PEF, daily symptoms and the need for ICS in patients using on-demand b2-agonists compared with patients using fenoterol regularly 4 times a day. In the group of patients who regularly took fenoterol, poor control over asthma symptoms was observed, in addition, there were more frequent and severe exacerbations compared to the group of patients using b2-agonists “on demand” for six months. In the latter, there was an improvement in indicators of pulmonary function, morning PEF, and a decrease in the response to a bronchoprovocation test with methacholine. An increase in bronchial hyperreactivity during regular use of short-acting b2-agonists is most likely due to the presence of S-enantomers in the racemic mixture of the drug.
With respect to salbutamol, similar patterns could not be established, although, as in the case of fenoterol, its regular use was accompanied by a slight increase in bronchial hyperreactivity. There is some evidence that regular use of salbutamol is accompanied by an increase in the frequency of episodes of AFU and an increase in the severity of inflammation in the DP.
Short-acting b 2 -agonists should be used (including as monotherapy) only “on demand”. It is unlikely that the commonly recommended on-demand dosing regimen of b2-agonists would worsen asthma control, but when high doses of the drug are used, deterioration of control becomes real. Moreover, many patients become especially sensitive to agonists in the presence of b 2 -adrenergic receptor polymorphism, which causes a more rapid deterioration of control. The connection established between the increased risk of death in patients with asthma and the use of high doses of inhaled b 2 -agonists reflects only the severity of the disease. It is also possible that high doses of inhaled b2-agonists have a deleterious effect on the course of asthma. Patients receiving high doses of b 2 -agonists (more than 1.4 aerosol cans per month) certainly need effective anti-inflammatory therapy, incl. and in order to reduce the dose of b 2 -agonists. When the need for bronchodilators increases (more than three times a week), additional prescription of anti-inflammatory drugs is indicated, and when using b2-agonists more than 3-4 times a day to relieve symptoms, an increase in their dose is indicated.
Taking short-acting b2-agonists for the purpose of bronchoprotection is also limited to “reasonable limits” (no more than 3-4 times a day). The bronchoprotective properties of b 2 -agonists allow many highly qualified athletes suffering from asthma to compete at international level (the rules allow the use of short-acting b 2 -agonists for the prevention of AFU, provided that the disease is medically verified). For example, at the 1984 Olympic Games in Los Angeles, 67 athletes with AFS took part, of which 41 received medals of various denominations. It is known that oral b 2 -agonists improve performance by increasing muscle mass, protein and lipid anabolism, and psychostimulation. In a study by S. Goubart et al. It has been shown that the effect of inhaled b 2 -agonists in healthy athletes is limited to only a slight bronchodilation, which, however, can make a significant contribution to improving respiratory adaptation at the beginning of exercise.
Long-acting inhaled b2-agonists
Currently available long-acting inhaled b 2 -agonists - formoterol and salmeterol - act within 12 hours with an equivalent bronchodilator effect. Nevertheless, there are differences between them. First of all, this is the speed of action of formoterol (in the form of DPI), comparable to the time of onset of action of salbutamol (in the form of MDI), which allows the use of formoterol as an emergency drug, instead of short-acting b 2 -agonists. At the same time, there are significantly fewer adverse events when using formoterol than when using salbutamol. These drugs can be used as monotherapy in patients with mild BA as bronchoprotectors in AFU. When using formoterol more than 2 times a week “on demand”, it is necessary to add ICS to the treatment.
It should be noted that monotherapy with long-acting b2-agonists is not recommended on a regular basis, as there is still no reliable evidence of their anti-inflammatory, disease-modifying effect.
There is scientifically based evidence of the advisability of the combined use of ICS and bronchodilators. Corticosteroids increase b2 receptor expression and reduce potential desensitization, while long-acting b2 agonists increase the sensitivity of corticosteroid receptors to ICS.
Studies conducted to date indicate the possibility of earlier administration of long-acting inhaled b 2 -agonists. For example, in patients with inadequate control of asthma while taking 400-800 mcg of ICS, additional administration of salmeterol provides more complete and adequate control compared to increasing the dose of ICS. Formoterol demonstrates a similar effect and at the same time helps to reduce the frequency of exacerbations of the disease. These and a number of other studies indicate that the addition of long-acting inhaled b2-agonists to low-moderate dose ICS therapy in patients with inadequate asthma control is equivalent to doubling the dose of steroids.
Currently, it is recommended to use long-acting inhaled b 2 -agonists only in patients simultaneously receiving ICS. Fixed-dose combinations such as salmeterol with fluticasone (Seretide) and formoterol with budesonide (Symbicort) appear promising. In this case, better compliance is noted, and the risk of using only one of the drugs as part of long-term therapy of the disease is eliminated.
Literature:1. National Institutes of Health, National Heart, Lung, and Blood Institute. Expert Panel Report 2: Guidelines for the Diagnosis and Management of Asthma. Bethesda, Md: National Institutes of Health, National Heart, Lung, and Blood Institute; April 1997. NIH publication 97-4051.
2. Lawrence D.R., Benitt P.N. Clinical pharmacology. In 2 volumes. Moscow: Medicine; 1991
3. Mashkovsky M.D. Medicines. Moscow: Medicine; 1984
4. Show M. B2-agonists, from pharmacological properties to everyday clinical practice. International workshop report (based on a workshop held in London, UK February 28-29, 200)
5. Barnes P.J. b -Agonists, Anticholinergics, and Other Nonsteroid Drugs. In: Albert R., Spiro S., Jett J., editors. Comprehensive Respiratory Medicine. UK:Harcourt Publishers Limited; 2001. p.34.1-34-10
6. Updating guidelines on asthma in adults (editorial). BMJ 2001; 323:1380–1381.
7. Jonson M. b 2 -adrenoceptor agonists: optimal pharmacological profile. In: The role of b2-agonists in asthma management. Oxford: The Medicine Group; 1993. p. 6-8.
8. Barnes P.J. beta-adrenergic receptors and their regulation. Am J Respir Crit Care Med. 1995; 152:838-860.
9. Kume H., Takai A., Tokuno H., Tomita T. Regulation of Ca 2 + dependent K+-channel activity in tracheal myocytes by phosphorylation. Nature 1989; 341:152-154.
10. Anderson G.P. Long acting inhaled beta-adrenoceptor agonists: the comparative pharmacology of formoterol and salmeterol. Agents Actions Suppl. 1993; 43:253-269.
11. Stiles GL, Taylor S, Lefkowitz RJ. Human cardiac beta-adrenergic receptors: subtype heterogeneity delineated by direct radioligand binding. Life Sci. 1983; 33:467-473.
12. Prior JG, Cochrane GM, Raper SM, Ali C, Volans GN. Self-poisoning with oral salbutamol. BMJ. 1981; 282:1932.
13. Handley D. The asthma-like pharmacology and toxicology of (S)-isomers of beta agonists. J Allergy Clin Immunol. 1999;104:S69-S76.
14. Johnson M., Coleman R. Mechanisms of action of beta-2-adrenoceptor agonists. In: Bisse W., Holgate S., editorials. Asthma and Rhinitis. Blackwell Science; 1995. p.1278-1308.
15. Burggsaf J., Westendorp R.G.J., in’t Veen J.C.C.M et al. Cardiovascular side effects of inhaled salbutamol in hypoxic asthmatic patients. Thorax 2001; 56: 567-569.
16. Van Shayck C.P., Bijl-Hoffland I.D., Closterman S.G.M. et al. Potential masking effect on dispnoea perception by short- and long-acting b 2 -agonists in asthma. ERJ 2002; 19:240-245.
17. Van der Woude H.J., Winter T.N., Aalbers R. Decreased bronchodilating effect of salbutamol in relieving methacholine induced moderate to severe bronchoconstriction during high dose treatment with long acting b 2 agonists. Thorax 2001; 56: 529-535.
18. Nelson HS. Clinical experience with levalbuterol. J Allergy Clin Immunol. 1999; 104:S77-S84.
19. Lipworth BJ, Hall IP, Tan S, Aziz I, Coutie W. Effects of genetic polymorphism on ex vivo and in vivo function of b 2 -adrenoceptors in asthmatic patients. Chest 1999;115:324-328.
20. Lipworth B.J., Kopelman G.H., Wheatley A.P. et al. b 2 -adrenoceptor promoter polymorphism: extended halotypes and functional effects in peripheral blood mononuclear cells. Thorax 2002; 57: 61-66.
21. Lima JJ, Thomason DB, Mohamed MH, Eberle LV, Self TH, Johnson JA. Impact of genetic polymorphisms of the b 2 -adrenergic receptor on albuterol bronchodilator pharmacodynamics. Clin Pharm Ther 1999; 65:519-525.
22. Kotani Y, Nishimura Y, Maeda H, Yokoyama M. b 2 -adrenergic receptor polymorphisms affect airway responsiveness to salbutamol in asthmatics. J Asthma 1999; 36:583-590.
23. Taylor D.R., Sears M.R., Cockroft D.W. The beta-agonist controversy. Med Clin North Am 1996; 80: 719-748.
24. Spitzer WO, Suissa S, Ernst P, et al. The use of beta-agonists and the risk of death and near death from asthma. N Engl J Med 1992; 326:501-506.
25. Sears MR, Taylor DR, Print CG, et al. Regular inhaled beta-agonist treatment in bronchial asthma. Lancet 1990; 336:1391–1396.
26. Handley D. The asthma-like pharmacology and toxicology of (S)-isomers of beta agonists. J Allergy Clin Immunol. 1999; 104:S69-S76.
27. Nelson HS. Clinical experience with levalbuterol. J Allergy Clin Immunol 1999;104:S77-S84.
28. Liggett S.B. Polymorphisms of the b 2 -adrenergic receptor in asthma. Am J Respir Cri. Care Med 1997; 156: S 156-162.
29. Voy R.O. The US Olympic Commitee experience with exercise-induced bronchospasm. Med Sci Exerc 1986; 18:328-330.
30. Lafontan M, Berlan M, Prud'hon M. Les agonistes beta-adrenergiques. Mecanismes d'action: lipomobilization et anabolism. Reprod Nutr Develop 1988; 28:61-84
31. Martineau L, Horan MA, Rothwell NJ, et al. Salbutamol, a b 2 -adrenoceptor agonist, increases skeletal muscle strength in young men. Clin Sci 1992; 83: 615-621.
32. Price AH, Clissold SP. Salbutamol in the 1980s. A reappraisal of its clinical efficacy. Drugs 1989; 38: 77-122.
33. Goubault C, Perault M-C, Leleu et al. Effects of inhaled salbutamol in exercising non-asthmatic athletes Thorax 2001; 56: 675-679.
34. Seberova E, Hartman P, Veverka J, et al. Formoterol given by Turbuhaler® had a rapid onset of action as salbutamol given by pMDI. Program and abstracts of the 1999 International Conference of the American Thoracic Society; April 23-28, 1999; San Diego, California. Abstract A637.
35. Wallin A., Sandstrom T., Soderberg M. et al. The effects of regularly inhaled formoterol, budesonide, and placebo on mucosal inflammation and clinical indicators of mild asthma. Am J Respir Crit Care Med. 1998; 158:79-86.
36. Greening AP, Ind PW, Northfield M, Shaw G. Added salmeterol versus higher-dose corticosteroid in asthma patients with symptoms on existing inhaled corticosteroid. Allen & Hanburys Limited UK Study Group. Lancet. 1994; 334:219-224.