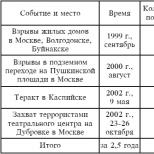
Pernicious anemia is a fatal disease that can be cured with a vitamin. Pernicious anemia - causes, symptoms, diagnosis and treatment Remedy for the treatment of pernicious anemia
The main functions of the blood, such as the transport of oxygen and nutrients to tissues and the removal of metabolic products, are carried out thanks to red blood cells - erythrocytes. When the number of these cells in the blood decreases, a pathological condition develops - anemia. According to the mechanism of development of anemic syndrome, there are three main factors - large blood loss, destruction of red blood cells in autoimmune diseases, and a decrease in the production of red blood cells in the body.
Let's consider one of the infrequent forms of pathology that develops as a result of impaired blood formation - pernicious anemia.
Pernicious anemia - what is it?
Pernicious anemia, or Addison-Birmer disease, develops when the synthesis of red blood cells (erythrocytes) is impaired due to a lack of vitamin B12 in the body. Cobalamin (B12) deficiency occurs as a result of insufficient intake of this substance or the body's inability to absorb it.
This disease is characterized by a disruption in the maturation of red blood cells in the bone marrow; their synthesis is interrupted at the stage of megaloblasts - immature blood cells that are large in size and contain an increased amount of hemoglobin. Megaloblasts are unable to perform the transport function of blood and are soon destroyed when passing through the spleen, which is why the body's cells can experience oxygen starvation, as well as intoxication with the products of their own decay.
In addition to the synthesis of red blood cells, cobalamin is involved in the oxidation of fatty acids and the utilization of their breakdown products; in a deficient state, this process stops and toxic substances accumulate in the body, destroying the sheath of nerve fibers. Addison-Birmer disease is the only anemia accompanied by neurological symptoms and mental disorder.
Due to the large size of the cells, anemia is called megaloblastic, and an increased hemoglobin content, which gives the cells a bright color, indicates hyperchromic pathology.
Manifestations of anemic syndrome were first described in 1855 by Thomas Addison, who was unable to find out the causes of the disease. A little later, the German doctor Anton Birmer studied the mechanism of development of anemia, giving it the name pernicious, which means “malignant”. In those days, malignant anemia was an incurable disease, over time leading to irreversible changes in internal organs, nervous exhaustion and even death. And only half a century later, a group of doctors made a discovery that was awarded the Nobel Prize; they were able to cure anemia in dogs by adding raw liver to food, and later isolate a factor from the liver that eliminates anemia, which was called vitamin B12 or extrinsic Castle factor.
The disease develops in 1% of people in the older age category. The risk group includes teenagers, athletes, and women in late pregnancy who need increased amounts of the vitamin. In children, pathology develops due to a hereditary predisposition to the disease; external factors can include serious malnutrition, as well as the mother’s vegetarianism during the period of bearing the baby.
Causes of the disease and risk factors
Vitamin B12 is synthesized by a special strain of bacteria and can only be absorbed in the lower part of the small intestine. In herbivores and some bird species, the intestinal microflora is populated by bacteria that produce cobalamin, which allows them to replenish the substance on their own. In the human body, such bacteria inhabit only the large intestine, so the vitamin B12 they synthesize is excreted along with the feces.
For this reason, a person can only obtain B12 from animal products, since plant foods contain its inactive analogue. Most cobalamin is found in the kidneys and liver, a little less in meat and seafood, dairy products and eggs contain small amounts of the vitamin, but if they are consumed regularly, a deficiency of this nutrient can be avoided.
Once in the stomach, vitamin B12 forms a bond with protein molecules (gastromucoprotein), which is synthesized by special cells of the gastric epithelium. This protein is commonly called intrinsic Castle factor; it protects cobalamin from the damaging effects of the acidic environment of the gastrointestinal tract. The breakdown of the protein-vitamin complex occurs in the small intestine, in its lower section, where the vitamin is absorbed by the mucous membrane and enters directly into the blood.
Pernicious anemia develops when one of the links that ensures the supply, absorption or storage of the vitamin in the human body is excluded. These may be the following factors:
- Insufficient intake or complete absence of foods containing vitamin B12 in the diet. Since cobalamin can accumulate in the liver and other organs, its reserves in the body are impressive; they can last for a couple of years, provided that animal products are completely avoided.
- Helminthic infestations. Infection with tapeworms that absorb vitamin B12.
- Disruption of the gastric epithelial cells responsible for the synthesis of gastromucoprotein, due to which the vitamin is destroyed without reaching the intestines. Factors contributing to the development of this pathology may include:
- taking medications that interfere with the fermentation of the stomach or changes in the cells of its mucous membrane;
- autoimmune diseases in which cells that produce internal Castle factor are degenerated;
- hereditary diseases, which are characterized by the absence of protective protein in the stomach or its slow synthesis;
- gastritis or;
- change in the acidity of gastric juice.
- Acute and chronic intestinal diseases in which the absorption of vitamin B12 is impaired, such as:
- malignant neoplasms;
- partial removal of the small intestine;
- Crohn's disease;
- intestinal dysbiosis;
- Zollinger-Ellison syndrome.
- Impaired storage of vitamin reserves in the liver when it is destroyed by cirrhosis.
Anemia can occur with increased consumption of the vitamin by the body during growth, significant strength loads, and multiple pregnancies. However, provided proper nutrition and the absence of other factors that contribute to the aggravation of the pathology, B12-deficiency anemia can self-correct.
What happens in the body when there is a lack of vitamin B12
Pernicious anemia has a gradual tendency to develop, manifesting itself first as an anemic syndrome, and then disrupting the functioning of the nervous system and internal organs.
Long before the appearance of neurological disorders and signs of megaloblastic anemia, the patient complains of weakness, drowsiness, severe headaches, loss of appetite, and dizziness. Such symptoms indicate oxygen starvation of cells caused by impaired transport of hemoglobin by red blood cells. A decrease in blood viscosity leads to changes in its pressure, which is expressed in arrhythmia and tachycardia.
Since immature red blood cells (megaloblasts) have a short lifespan, their death and breakdown of hemoglobin in the liver and spleen leads to an increase in these organs and disruption of the functioning of these organs.
Over time, the skin and sclera of the eyes may become jaundiced due to incomplete removal of bilirubin by the liver, and the tissues of the tongue, accumulating hemoglobin, become inflamed. A characteristic sign of pernicious anemia is an enlarged tongue, scarlet in color with atrophied papillary epithelium, due to which the organ becomes smooth.
The progression of the disease leads to damage to the epithelium of the oral cavity and gastrointestinal tract, which is expressed in the following symptoms:
- stomatitis and burning sensation of the tongue;
- glossitis - inflammation of the tissues of the tongue;
- feeling after eating;
- chronic constipation;
- pain in the intestines.
When fatty acid metabolism is disturbed, toxic substances accumulate, destroying the fatty membrane of neurons in the brain and spinal cord. CNS lesions manifest themselves as follows:
- memory loss;
- disorientation;
- absent-mindedness;
- irritability.
The long-term course of B12 deficiency anemia syndrome can be manifested by behavioral disorders, inability to formulate and express thoughts, and memory loss. Since the ability to absorb the vitamin decreases with age, older people are most in need of additional sources of cyanocobalamin. The symptoms of Addison-Birmer disease are often confused with senile dementia, but the disease is so easy to cure.
With degenerative damage to the spinal cord, funicular myelosis occurs, which is characterized by the following symptoms:
- numbness of the limbs, which is accompanied by tingling;
- convulsions;
- unsteady gait, stiffness and weakness in the legs;
- loss of sensation in the feet.
In later stages, manifestations of the disease may include:
- urinary disturbance;
- sexual dysfunction in men;
- decreased hearing and vision;
- mental disorders;
- hallucinations;
- paresis and paralysis;
- amyotrophy.
Diagnosis and differential diagnosis
The diagnosis of pernicious anemia is made based on the following indications:
- collection of patient complaints, from which the doctor can determine the duration of the disease;
- a physical examination of the patient during which the doctor pays attention to changes in the epithelial covers of the tongue, skin tone, and decreased sensitivity of the extremities.
- lab tests.
Mandatory laboratory tests for suspected B12-deficiency anemia are:
- Clinical blood test. With cyanocobalamin deficiency, red blood cells have an increased size, pronounced color, and uneven shape. The values of leukocytes, erythrocytes and platelets in the blood are reduced, while the values of lymphocytes exceed the norm.
- Immunological analysis for the presence of antibodies to intrinsic Castle factor in the blood.
- Bone marrow analysis performed by puncture shows the megaloblastic type of hematopoiesis.
- Urine and stool tests are necessary to determine the amount of vitamin B12 that is excreted from the body.
- If the amount of cyanocobalamin is increased in the analysis, the Schilling test is performed to determine the cause of poor absorption of the substance.
Additional diagnostics can help determine the cause of anemia. Thus, gastroscopy allows you to determine the content of hydrochloric acid in the stomach, as well as the presence of antibodies that destroy stomach cells that synthesize protective protein. Additionally, a stool test is prescribed to check for the presence of helminthic infestations in the body. Studies of the stomach, intestines and liver are carried out if pathological diseases are suspected that have led to the development of anemia.
When making a diagnosis, Addison-Biermer disease is differentiated from erythromyelosis and folate deficiency anemia.
Treatment of Addison-Biermer disease
Treatment of pernicious anemia is carried out under the supervision of such specialists as a hematologist, gastroenterologist, and neurologist.
The main therapy consists of replenishing the deficiency of vitamin B12 in the body by administering it subcutaneously. At the same time, the gastrointestinal tract is treated, the microflora is normalized, and, if necessary, helminthic infestation is eliminated. For autoimmune pathologies, glucocorticosteroids are administered simultaneously with synthetic vitamin preparations to neutralize antibodies to the intrinsic factor.
Drug treatment with drugs "Oxycobalamin" or "Cyanocobalamin", which are administered in the form of subcutaneous injections, takes place in two stages - saturation and maintenance. During an exacerbation, the patient is administered the drug daily; the dosage and duration of the course depend on the age and severity of anemia. After vitamin B12 levels have returned to normal, maintenance therapy is carried out, which consists of administering the drug once every two weeks.
In parallel with this, diet therapy is used, which consists in correcting the patient’s diet. Foods rich in vitamin B12 are introduced into the daily diet, for example, beef, pork and chicken liver, seafood, mackerel, sardines, and dairy products.
The timing of complete restoration of hematopoiesis depends on the initial severity of anemia. Improvement occurs 2–3 months after the start of therapy.
Treatment prognosis and possible complications
With timely treatment, the symptoms of the pathology are gradually eliminated, the skin acquires a natural shade after 2 weeks, after the red blood cell rate has been restored, digestive problems disappear, and stool returns to normal. Neurological disorders are gradually smoothed out, tissue sensitivity is normalized, gait is restored, neuropathy and memory loss disappear.
Unfortunately, if the stage is too advanced, the atrophied optic nerves, as well as the leg muscles, cannot be restored. In very rare cases, after recovery, patients develop toxic goiter and myxedema.
If anemia occurs during pregnancy, vitamin B12 deficiency leads to placental abruption and premature birth. Insufficient oxygen supply to the fetus causes hypoxia (oxygen starvation), which affects the growth and development of the child.
In children with a hereditary vitamin B12 absorption disorder, anemia can manifest itself in enlarged internal organs (liver and spleen), decreased appetite, and developmental delays. The cause of cyanocobalamin deficiency in babies may be the mother's vegetarianism during breastfeeding.
Prevention
To prevent the development of the disease, you should properly organize your diet by including animal products rich in vitamin B12. Fatty foods should be limited, as they slow down the process of hematopoiesis. You should also not abuse medications that inhibit the production of hydrochloric acid in gastric juice and can cause the destruction of the vitamin.
For chronic diseases of the stomach and liver, you should regularly undergo a blood test to determine the vitamin content in the body.
Elderly people need to take vitamin B12 as part of multivitamin complexes, or take injections of the drug for preventive purposes.
Pernicious anemia is a serious disease that can lead to irreversible changes in the body and even disability. Before the discovery of vitamin B12 and Castle factor, the pathology was considered incurable and caused a slow decline, ending in death. Nowadays, the disease is very rare and occurs mainly due to impaired absorption of the vitamin with concomitant gastrointestinal diseases. However, people who practice veganism (strict vegetarianism), as well as those who practice therapeutic fasting, put themselves at risk of developing B12-deficiency anemia.
My name is Elena. Medicine is my calling, but it so happened that I was unable to realize my desire to help people. But, I am the mother of three beautiful children, and writing articles on medical topics has become my hobby. I want to believe that my texts are understandable and useful to the reader.
Blood picture: hyperchromic type anemia, macrocytes, megalocytes, with Jolly bodies, Cabot rings, leukopenia (during exacerbation).
Treatment 100-200 mcg is administered intramuscularly daily or every other day before the onset. If an anemic coma occurs, urgent hospitalization is recommended, preferably red blood cell mass (150-200 ml). Maintenance therapy with vitamin B12 is necessary to prevent relapses. Systematic monitoring of blood composition in people with persistent achylia, as well as those who have suffered, is indicated. Patients suffering from pernicious anemia should be under medical supervision (stomach cancer may occur).
1. Malignant anemia(synonym: pernicious anemia, Addison-Biermer disease). Etiology and pathogenesis. Currently, pernicious anemic syndrome is considered as a manifestation of B12-avitaminosis, and Addison-Beermer disease is considered as endogenous B12-avitaminosis due to atrophy of the fundic glands that produce gastromucoprotein, resulting in impaired absorption of vitamin B1a, necessary for normal, normoblastic, hematopoiesis, and Pathological, megaloblastic, hematopoiesis develops, leading to anemia of the “pernicious” type.
Clinical picture (symptoms and signs). Persons over the age of 40-45 years become ill. Characterized by disorders of the cardiovascular, nervous, digestive and hematopoietic systems. The complaints of patients are varied: general weakness, shortness of breath, palpitations, pain in the heart, swelling of the legs, dizziness, a crawling sensation in the hands and feet, gait disorder, burning pain in the tongue and esophagus, periodic diarrhea. The patient's appearance is characterized by pale skin with a lemon-yellow tint. The sclera is subicteric. The patients are not exhausted. The face is puffy, swelling in the ankles and feet. Edema can reach large degrees and be accompanied by ascites and hydrothorax. From the cardiovascular system - the appearance of systolic murmur at all orifices of the heart and the “spinning top” murmur at the bulb of the jugular vein, which is associated with a decrease in blood viscosity and acceleration of blood flow; Anoxemic angina is possible. With prolonged anemia, fatty degeneration of organs develops, including the heart (“tiger heart”), as a result of persistent anoxemia. On the part of the digestive organs - the so-called Hunter's (Hunter's) glossitis, the tongue is clean, bright red, devoid of papillae. Analysis of gastric juice usually reveals histamine-resistant achylia. Periodic diarrhea is a consequence of enteritis. The liver is enlarged and soft; in some cases - a slight enlargement of the spleen. If the number of red blood cells drops significantly (below 2,000,000), a fever of the wrong type is observed. Changes in the nervous system are associated with degeneration and sclerosis of the posterior and lateral columns of the spinal cord (funicular myelosis). The clinical picture of the nervous syndrome consists of combinations of spastic spinal paralysis and tabic symptoms (the so-called pseudotabes): spastic paraparesis with increased and pathological reflexes, clonus, crawling sensation, numbness of the limbs, girdle pain, impaired vibration and deep sensitivity, sensory ataxia and dysfunction pelvic organs; less commonly, bulbar phenomena.
Blood picture. The most characteristic symptom is anemia of the hyperchromic type. The morphological substrate of hyperchromia is large, hemoglobin-rich erythrocytes - macrocytes and megalocytes (the latter reach 12-14 microns or more). As the disease worsens, the number of reticulocytes in the blood sharply decreases. The appearance of a large number of reticulocytes portends imminent remission.
An exacerbation of the disease is characterized by the appearance of degenerative forms of erythrocytes [poikilocytes, schizocytes, basophilic punctured erythrocytes, erythrocytes with Jolly bodies and Cabot rings (color table, Fig. 3)], individual megaloblasts (color table, Fig. 5). Changes in white blood are characterized by leukopenia due to a decrease in the number of cells of bone marrow origin - granulocytes. Among the cells of the neutrophil series, giant, polysegmented neutrophils are found. Along with a shift of neutrophils to the right, a shift to the left is observed with the appearance of juvenile forms and even myelocytes. The number of platelets during an exacerbation is significantly reduced (to 30,000 or less), but thrombocytopenia, as a rule, is not accompanied by hemorrhagic phenomena.
Bone marrow hematopoiesis during the period of exacerbation of pernicious anemia occurs according to the megaloblastic type. Megaloblasts are a morphological expression of a kind of “dystrophy” of bone marrow cells in conditions of insufficient supply of a specific factor - vitamin B12. Under the influence of specific therapy, normoblastic hematopoiesis is restored (color table, Fig. 6).
Symptoms of the disease develop gradually. Many years before the disease, gastric achylia is detected. At the onset of the disease, general weakness is noted; patients complain of dizziness and palpitations at the slightest physical exertion. Then dyspeptic symptoms and paresthesia are added; patients consult a doctor, already in a state of significant anemia. The course of the disease is characterized by cyclicity - alternating periods of improvement and deterioration. In the absence of proper treatment, relapses become increasingly prolonged and severe. Before the introduction of liver therapy into practice, the disease fully justified its name “disastrous” (pernicious). During the period of severe relapse - severe anemia and rapid progression of all symptoms of the disease - a life-threatening coma (coma perniciosum) may develop.
Pathological anatomy. An autopsy of a person who died from pernicious anemia reveals severe anemia of all organs, with the exception of the red bone marrow; the latter, being in a state of hyperplasia, fills the diaphyses of the bones (color table, Fig. 7). Fatty infiltration of the myocardium (“tiger heart”), kidneys, and liver is noted; in the liver, spleen, bone marrow, lymph nodes - hemosiderosis (color table, Fig. 8). Changes in the digestive organs are characteristic: the papillae of the tongue are atrophic, atrophy of the gastric mucosa and its glands is adenia. In the posterior and lateral columns of the spinal cord, very characteristic degenerative changes are noted, referred to as combined sclerosis, or funicular myelosis.
Rice. 3. Blood in anemia: 1 - 4 - erythrocytes of the last stage of normal hematopoiesis (conversion of erythroblast into erythrocyte); 5 - 9 - nuclear disintegration with the formation of Jolly bodies in basophilically punctured (5, 6) and polychromatophilic (7 - 9) erythrocytes; 10 and 11- Jolly bodies in orthochromic erythrocytes; 12 - chromatin particles in erythrocytes; 13 - 16 - Cabot rings in basophilically punctured (13, 14) and orthochromic (15, 16) erythrocytes (pernicious anemia); 17 - 23 - basophilic punctured erythrocytes in lead anemia; 24 and 25 - polychromatophilic erythrocytes (microcyte and macrocyte); megalocyte (26) and poikilocyte (27) in pernicious anemia; 28 - normocyte; 29 - microcytes.

Rice. 5. Blood in pernicious anemia (severe relapse): orthochromic (1) and polychromatophilic (2) megalocytes, erythrocytes with Cabot rings (3), Jolly bodies (4) with basophilic punctuation (5), megaloblasts (6), polysegmented neutrophil (7) , anisocytosis and poikilocytosis (8).

Rice. 6. Bone marrow in pernicious anemia (initial remission 24 hours after administration of 30 mcg of vitamin B12): 1 - normoblasts; 2 - metamyelocytes; 3 - band neutrophil; 4 - erythrocyte.

Rice. 7. Myeloid hyperplasia of the bone marrow in malignant anemia.
Rice. 8. Hemosiderin pigmentation of the periphery of the hepatic lobules in pernicious anemia (reaction to Prussian blue).
Treatment. Since the 20s, raw liver, especially lean veal liver, minced through a meat grinder (200 g per day), has been used with great success for the treatment of malignant anemia. A great achievement in the treatment of pernicious anemia was the production of liver extracts, especially for parenteral administration (campolon, antianemin). The specificity of the action of liver drugs in pernicious anemia is due to the content of vitamin B12 in them, which stimulates the normal maturation of erythroblasts in the bone marrow.
The greatest effect is achieved with parenteral use of vitamin B12. The daily dose of vitamin B2 is 50-100 mcg. The drug is administered intramuscularly depending on the patient's condition - daily or every 1-2 days. Oral administration of vitamin B12 is effective only in combination with the simultaneous intake of internal antianemic factor (gastromucoprotein). Currently, favorable results have been obtained from the treatment of patients with pernicious anemia through the internal use of the drug mucovita (available in the form of pills) containing vitamin B12 (200-500 mcg each) in combination with gastromucoprotein (0.2). Mukovit is prescribed 3-6 tablets per day daily until the onset of reticulocyte crisis and then 1-2 times a day until the onset of hematological remission.
The immediate effect of anti-anemic therapy in the sense of replenishing the blood with newly formed red blood cells begins to affect from the 5-6th day of treatment with an increase in reticulocytes to 20-30% and higher (“reticulocyte crisis”). Following the reticulocyte crisis, the amount of hemoglobin and red blood cells begins to increase, which reaches normal levels after 3-4 weeks.
Folic acid, administered orally or parenterally at a dose of 30-60 mg or more (up to 120-150 mg) per day, causes a rapid onset of remission, but does not prevent the development of funicular myelosis. For funicular myelosis, vitamin B12 is used intramuscularly in large doses of 200-400 mcg, in severe cases, 500-000 (!) mcg per day] until complete clinical remission is achieved. The total dose of vitamin B12 during a 3-4 week course of treatment for anemia is 500-1000 mcg, for funicular myelosis - up to 5000-10,000 mcg and higher.
The effectiveness of vitamin B12 therapy has a known limit, after which the increase in blood counts stops and anemia becomes hypochromic; During this period of illness, it is advisable to use treatment with iron preparations (2-3 g per day, washed down with diluted hydrochloric acid).
The issue of using blood transfusions for pernicious anemia is decided in each case according to indications. An absolute indication is pernicious coma, which poses a threat to life due to increasing hypoxemia. Repeated blood transfusions or (better) red blood cells (250-300 ml each) often save the lives of patients until the therapeutic effect of vitamin B12 manifests itself.
Prevention. The minimum daily human requirement for vitamin B12 is 3-5 mcg, therefore, in order to prevent relapse of pernicious anemia, it can be recommended to inject 100-200 mcg of vitamin B12 2 times a month, and in spring and autumn (when relapses occur more often) - once a week or 10 days. It is necessary to systematically monitor the blood composition of persons who have undergone extensive gastrectomy, as well as those who have persistent gastric achylia, provide them with a nutritious diet, and, if necessary, apply early antianemic treatment. It should be remembered that pernicious anemia can be an early symptom of stomach cancer. In general, it is known that patients with gastric achylia and especially pernicious anemia are more likely than others to develop stomach cancer. Therefore, all patients with pernicious anemia should be under clinical observation and undergo an annual control X-ray examination of the stomach.
The disease, described by Addison in 1855 and Biermer in 1868, became known among doctors as pernicious anemia, that is, a fatal, malignant disease. Only in 1926, in connection with the discovery of hepatic therapy for pernicious anemia, the idea that had prevailed for a century about the absolute incurability of this disease was refuted.
Clinic. People over 40 years of age usually get sick. The clinical picture of the disease consists of the following triad: 1) disorders of the digestive tract; 2) disorders of the hematopoietic system; 3) disorders of the nervous system.
Symptoms of the disease develop unnoticed. Already many years before the pronounced picture of malignant anemia, gastric achylia is detected, and in rare cases, changes in the nervous system are noted.
At the onset of the disease, increasing physical and mental weakness appears. Patients quickly get tired, complain of dizziness, headaches, tinnitus, “flying spots” in the eyes, as well as shortness of breath, palpitations at the slightest physical exertion, drowsiness during the day and night insomnia. Then dyspeptic symptoms (anorexia, diarrhea) occur, and patients consult a doctor already in a state of significant anemia.
Other patients initially experience pain and a burning sensation in the tongue, and they turn to specialists in oral diseases. In these cases, one examination of the tongue, revealing signs of typical glossitis, is sufficient to make the correct diagnosis; the latter is supported by the anemic appearance of the patient and the characteristic blood picture. The symptom of glossitis is very pathognomonic, although not strictly specific for Addison-Biermer disease.
Relatively rarely, according to various authors, in 1-2% of cases, pernicious anemia begins with symptoms of angina pectoris provoked by myocardial anoxemia. Sometimes the disease begins as a nervous disease. Patients are concerned about paresthesia - a feeling of crawling, numbness in the distal parts of the extremities or radicular pain.
The appearance of the patient during an exacerbation of the disease is characterized by severe pallor of the skin with a lemon-yellow tint. The sclera is subicteric. Often the integument and mucous membranes are more icteric than pale. Brown pigmentation in the form of a “butterfly” is sometimes observed on the face - on the wings of the nose and above the cheek bones. The face is puffy, and swelling in the ankles and feet is quite common. Patients are usually not emaciated; on the contrary, they are well nourished and prone to obesity. The liver is almost always enlarged, sometimes reaching significant sizes, insensitive, and soft in consistency. The spleen has a denser consistency and is usually difficult to palpate; splenomegaly is rarely observed.
The classic symptom - Hunter's glossitis - is expressed in the appearance of bright red areas of inflammation on the tongue, very sensitive to food intake and medications, especially acidic ones, causing the patient a burning sensation and pain. Areas of inflammation are most often localized along the edges and at the tip of the tongue, but sometimes they involve the entire tongue (“scalded tongue”). Aphthous rashes and sometimes cracks are often observed on the tongue. Such changes can spread to the gums, buccal mucosa, soft palate, and in rare cases to the mucous membrane of the pharynx and esophagus. Subsequently, the inflammatory phenomena subside and the papillae of the tongue atrophy. The tongue becomes smooth and shiny (“varnished tongue”).
Patients have a capricious appetite. Sometimes there is an aversion to food, especially meat. Patients complain of a feeling of heaviness in the epigastric region, usually after eating.
X-rays often reveal smoothness of the folds of the gastric mucosa and accelerated evacuation.
Gastroscopy reveals nested, less often total atrophy of the gastric mucosa. A characteristic symptom is the presence of so-called pearlescent plaques - shiny, mirror-like areas of mucosal atrophy, localized mainly in the folds of the gastric mucosa.
Analysis of gastric contents usually reveals achylia and increased mucus content. In rare cases, free hydrochloric acid and pepsin are contained in small quantities. Since the introduction of histamine testing into clinical practice, cases of pernicious anemia with preserved free hydrochloric acid in the gastric juice have become more common.
The Singer test, a rat-reticulocyte reaction, usually gives a negative result: the gastric juice of a patient with pernicious anemia, when administered subcutaneously to a rat, does not cause an increase in the number of reticulocytes, which indicates the absence of an internal factor (gastromucoprotein). Glandular mucoprotein is not detected even with special research methods.
The histological structure of the gastric mucosa obtained by biopsy is characterized by thinning of the glandular layer and a decrease in the glands themselves. The chief and parietal cells are atrophic and replaced by mucous cells.
These changes are most pronounced in the fundus, but can affect the entire stomach. Conventionally, three degrees of mucosal atrophy are distinguished: in the first degree, simple achlorhydria is noted, in the second, the disappearance of pepsin, in the third, complete achylia, including the absence of gastromucoprotein secretion. With pernicious anemia, the third degree of atrophy is usually observed, but there are exceptions.
Gastric achylia, as a rule, persists during remission, thereby acquiring a certain diagnostic value during this period. Glossitis may disappear during remission; its appearance portends an exacerbation of the disease.
The enzymatic activity of the intestinal glands, as well as the pancreas, is reduced.
During periods of exacerbation of the disease, enteritis with abundant, intensely colored feces is sometimes observed, which is caused by an increased content of stercobilin - up to 1500 mg in daily quantities.
Due to anemia, an anoxic state of the body develops, which primarily affects the circulatory and respiratory systems. Functional myocardial failure in pernicious anemia is caused by impaired nutrition of the heart muscle and its fatty degeneration.
The electrocardiogram shows symptoms of myocardial ischemia - a negative T wave in all leads, low voltage, widening of the ventricular complex. During the period of remission, the electrocardiogram takes on a normal appearance.
The temperature during the period of relapse often rises to 38°C or higher, but is more often subfebrile. The increase in temperature is mainly associated with the process of increased breakdown of red blood cells.
Changes in the nervous system are very important in diagnostic and prognostic terms. The pathomorphological basis of the nervous syndrome is degeneration and sclerosis of the posterior and lateral columns of the spinal cord, or the so-called funicular myelosis. The clinical picture of this syndrome consists of combinations of spastic spinal paralysis and tabetic symptoms. The first include: spastic paraparesis with increased reflexes, clonus and pathological reflexes of Babinsky, Rossolimo, Bekhterev, Oppenheim. Symptoms simulating tabes dorsalis (“pseudotabes”) include: paresthesia (crawling sensation, numbness of the distal limbs), girdle pain, hypotension and decreased reflexes up to areflexia, impaired vibration and deep sensitivity, sensory ataxia and dysfunction of the pelvic organs. .
Sometimes symptoms of damage to the pyramidal tracts or posterior columns of the spinal cord dominate; in the latter case, a picture is created that resembles a tabes. In the most severe, rare forms of the disease, cachexia develops with paralysis, complete loss of deep sensitivity, areflexia, trophic disorders and disorders of the pelvic organs (our observation). More often we see patients with initial symptoms of funicular myelosis, expressed in paresthesia, radicular pain, mild disturbances of deep sensitivity, unsteady gait and a slight increase in tendon reflexes.
Less commonly observed are lesions of the cranial nerves, mainly the visual, auditory and olfactory nerves, resulting in corresponding symptoms from the sensory organs (loss of smell, decreased hearing and vision). A characteristic symptom is central scotoma, accompanied by loss of vision and quickly disappearing under the influence of treatment with vitamin B12 (S. M. Ryse). In patients with pernicious anemia, peripheral neuron damage also occurs. This form, designated as polyneuritic, is caused by degenerative changes in various nerves - sciatic, median, ulnar, etc. or individual nerve branches.
Mental disorders are also observed: delusions, hallucinations, sometimes psychotic phenomena with depressive or manic moods; Dementia is more common in old age.
During the period of severe relapse of the disease, a coma (coma perniciosum) may occur - loss of consciousness, drop in temperature and blood pressure, shortness of breath, vomiting, areflexia, involuntary urination. There is no strict relationship between the development of comatose symptoms and a drop in red blood counts. Sometimes patients with 10 units of hemoglobin in the blood do not fall into a coma, but sometimes coma develops with 20 units or more of hemoglobin. In the pathogenesis of pernicious coma, the main role is played by the rapid pace of anemia, leading to severe ischemia and hypoxia of the centers of the brain, in particular the region of the third ventricle (A. F. Korovnikov).
Rice. 42. Hematopoiesis and blood destruction in pernicious B12 (folate) deficiency anemia.
Picture of blood. At the center of the clinical picture of the disease are changes in the hematopoietic system, leading to the development of severe anemia (Fig. 42).
The result of impaired bone marrow hematopoiesis is a kind of anemia, which during the period of relapse of the disease reaches an extremely high degree: observations are known when (with a favorable outcome!) hemoglobin decreased to 8 units (1.3 g%), and the number of red blood cells - to 140,000.
No matter how low hemoglobin decreases, the number of red blood cells drops even lower, as a result of which the color index always exceeds one, in severe cases reaching 1.4-1.8.
The morphological substrate of hyperchromia is large, hemoglobin-rich erythrocytes - macrocytes and megalocytes. The latter, reaching a diameter of 12-14 microns and more, are the end product of megaloblastic hematopoiesis. The apex of the erythrocytometric curve is shifted to the right from normal.
The volume of a megalocyte is 165 μm 3 or more, i.e., 2 times the volume of a normocyte; Accordingly, the hemoglobin content in each individual megalocyte is significantly higher than normal. Megalocytes are somewhat oval or elliptical in shape; they are intensely colored and do not show central clearing (Tables 19, 20).
During the period of relapse, degenerative forms of erythrocytes are observed - basophilically punctured erythrocytes, schizocytes, poikilocytes and microcytes, erythrocytes with preserved remnants of the nucleus in the form of Jolly bodies, Cabot rings, etc., as well as nuclear forms - erythroblasts (megaloblasts). More often these are orthochromic forms with a small pyknotic nucleus (incorrectly designated “normoblasts”), less often - polychromatophilic and basophilic megaloblasts with a nucleus of a typical structure.
The number of reticulocytes during an exacerbation is sharply reduced.
The appearance of reticulocytes in the blood in large quantities portends an imminent remission.
Changes in white blood are no less characteristic of pernicious anemia. During a relapse of pernicious anemia, leukopenia (up to 1500 or less), neutropenia, eosinopenia or aneosinophilia, abasophilia and monopenia are observed. Among the cells of the neutrophil series, a “shift to the right” is noted with the appearance of peculiar giant polysegmented forms containing up to 8-10 nuclear segments. Along with a shift of neutrophils to the right, a shift to the left is also observed with the appearance of metamyelocytes and myelocytes. Among monocytes there are young forms - monoblasts. Lymphocytes in pernicious anemia do not change, but their percentage is increased (relative lymphocytosis).
Table 19. Pernicious anemia. Blood picture in severe relapse of the disease. In the field of view, megaloblasts of various generations, megalocytes, erythrocytes with nuclear derivatives (Cabot rings, Jolly bodies) and basophilic punctation, a characteristic polysegmented neutrophil are visible.
Table 20. Pernicious anemia. The blood picture is in remission. Macroanisocytosis of erythrocytes, polysegmented neutrophil.
The number of blood platelets during an exacerbation is slightly reduced. In some cases, thrombocytopenia is observed - up to 30,000 or less. Platelets may be atypical in size; their diameter reaches 6 microns or more (so-called megaplatelet); Degenerative forms also occur. Thrombocytopenia in pernicious anemia is usually not accompanied by hemorrhagic syndrome. Only in rare cases are bleeding phenomena observed.
Bone marrow hematopoiesis. The picture of bone marrow hematopoiesis in pernicious anemia is very dynamic (Fig. 43, a, b; table 21, 22).
During the period of exacerbation of the disease, bone marrow puncture macroscopically appears abundant, bright red, which contrasts with the pale, watery appearance of peripheral blood. The total number of nucleated bone marrow elements (myelokaryocytes) is increased. The ratio between leukocytes and erythroblasts leuco/erythro instead of 3:1-4:1 normally becomes equal to 1:2 and even 1:3; therefore, there is an absolute predominance of erythroblasts.
Rice. 43. Hematopoiesis in pernicious anemia.
a - bone marrow punctate of a patient with pernicious anemia before treatment. Erythropoiesis occurs according to the megaloblastic type; b - bone marrow punctate of the same patient on the 4th day of treatment with liver extract (orally). Erythropoiesis occurs according to the macronormoblastic type.
In severe cases, in untreated patients, with pernicious coma, erythropoiesis occurs entirely according to the megaloblastic type. There are also so-called reticulomegaloblasts - cells of the reticular type of irregular shape, with wide pale blue protoplasm and a nucleus of a delicate cellular structure, located somewhat eccentrically. Apparently, megaloblasts in pernicious anemia can originate from both hemocytoblasts (via the erythroblast stage) and from reticular cells (return to embryonic angioblastic erythropoiesis).
The quantitative relationships between megaloblasts of different degrees of maturity (or different “ages”) are very variable. The predominance of promegaloblasts and basophilic megaloblasts in the sternal punctate creates a picture of “blue” bone marrow. In contrast, the predominance of fully hemoglobinized, oxyphilic megaloblasts gives the impression of “red” bone marrow.
A characteristic feature of megaloblastic cells is the early hemoglobinization of their cytoplasm while the delicate structure of the nucleus is still preserved. The biological feature of megaloblasts is anaplasia, i.e. loss by a cell of its inherent ability for normal, differentiating development and eventual transformation into an erythrocyte. Only a small part of megaloblasts mature to the final stage of their development and turn into anucleate megalocytes.
Table 21. Megaloblasts in the bone marrow in pernicious anemia (color microphoto).
Table 22. Pernicious anemia in the advanced stage of the disease (bone marrow punctate).
Below at 7 o'clock there is a promyelocyte, at 5 o'clock there is a characteristic hypersegmented neutrophil. All other cells are megaloblasts in various phases of development, starting from a basophilic promegaloblast with nucleoli (at 6 o'clock) and ending with an orthochromic megaloblast with a pyknotic nucleus (at 11 o'clock). Among megaloblasts, mitoses produce two- and three-nucleated cells.
Cellular anaplasia in malignant anemia has features in common with cellular anaplasia in malignant neoplasms and leukemia. Morphological similarity with blastoma cells is especially evident in polymorphonuclear, “monstrous” megaloblasts. A comparative study of the morphological and biological characteristics of megaloblasts in malignant anemia, hemocytoblasts in leukemia, and cancer cells in malignant neoplasms led us to the idea of a possible commonality of pathogenetic mechanisms in these diseases. There is reason to think that both leukemia and malignant neoplasms, like malignant anemia, arise under conditions of a deficiency of specific factors necessary for the normal development of cells in the body.
Megaloblasts are a morphological expression of a kind of “dystrophy” of the red nuclear cell, which “lacks” a specific maturation factor - vitamin B 12. Not all cells of the red row are anaplastic to the same extent; some of the cells appear as if in the form of transitional cells between normo- and megaloblasts ; these are the so-called macronormoblasts. These cells, which present particular difficulties for differentiation, are usually found in the initial stage of remission. As remission progresses, normoblasts come to the fore, and cells of the megaloblastic series recede into the background and completely disappear.
Leukopoiesis during an exacerbation is characterized by a delay in the maturation of granulocytes and the presence of giant metamyelocytes and polymorphonuclear neutrophils, the size of which is 2 times larger than that of normal neutrophils.
Similar changes - impaired ripening and pronounced nuclear polymorphism - are also observed in giant cells of the bone marrow. Both in immature megakaryocytes and in “overripe”, polymorphic forms, the processes of formation and release of platelets are disrupted. Megaloblastosis, polysegmented neutrophils and megakaryocyte changes are dependent on the same cause. This reason is a deficiency of a specific hematopoietic factor - vitamin B12.
Bone marrow hematopoiesis in the stage of hematological remission, in the absence of anemic syndrome, occurs according to the normal (normoblastic) type.
Increased breakdown of erythrocytes, or erythrorrhexis, occurs throughout the reticulohistiocytic system, including in the bone marrow itself, where some of the hemoglobin-containing erythromegaloblasts undergo the process of karyo- and cytorexis, which results in the formation of erythrocyte fragments - schizocytes. The latter partly enter the blood, partly are captured by phagocytic reticular cells - macrophages. Along with the phenomena of erythrophagy, significant accumulations of iron-containing pigment - hemosiderin, derived from the hemoglobin of destroyed red blood cells, are found in the organs.
Increased breakdown of erythrocytes does not give grounds to classify pernicious anemia as a hemolytic anemia (as was assumed by older authors), since erythrorrhexis occurring in the bone marrow itself is caused by defective hematopoiesis and is secondary in nature.
The main signs of increased breakdown of erythrocytes in pernicious anemia are icteric coloration of the integument and mucous membranes, enlarged liver and spleen, intensely colored golden blood serum with an increased content of “indirect” bilirubin, the constant presence of urobilin in the urine and pleiochromia of bile and feces with a significant increase in the content of stercobilin in kale.
Pathological anatomy. Thanks to the successes of modern therapy, pernicious anemia in the section is now very rare. When autopsying a corpse, one notices the anemia of all organs while maintaining fatty tissue. Fatty infiltration of the myocardium (“tiger heart”), kidneys, and liver is noted, and central fatty necrosis of the lobules is also found in the latter.
In the liver, spleen, bone marrow, lymph nodes, especially retroperitoneal ones, a significant deposition of fine-grained yellow-brown pigment - hemosiderin, which gives a positive reaction to iron, is determined. Hemosiderosis is more pronounced in Kupffer cells along the periphery of the hepatic lobules, while in the spleen and bone marrow hemosiderosis is much less pronounced, and sometimes does not occur at all (contrary to what is observed with true hemolytic anemia). A lot of iron is deposited in the convoluted tubules of the kidneys.
Changes in the digestive organs are very characteristic. The tongue papillae are atrophic. Similar changes can be observed in the mucous membrane of the pharynx and esophagus. In the stomach, atrophy of the mucous membrane and its glands is detected - anadenia. A similar atrophic process occurs in the intestines.
In the central nervous system, mainly in the posterior and lateral columns of the spinal cord, degenerative changes are noted, referred to as combined sclerosis or funicular myelosis. Less commonly, ischemic foci with necrotic softening of the nervous tissue are found in the spinal cord. Necrosis and foci of glial proliferation in the cerebral cortex have been described.
A typical sign of pernicious anemia is crimson-red, juicy bone marrow, which sharply contrasts with the general pallor of the integument and anemia of all organs. Red bone marrow is found not only in flat bones and epiphyses of long bones, but also in the diaphysis of the latter. Along with bone marrow hyperplasia, extramedullary foci of hematopoiesis (accumulation of erythroblasts and megaloblasts) are observed in the splenic pulp, liver and lymph nodes. Reticulo-histiocytic elements in the hematopoietic organs and extramedullary foci of hematopoiesis exhibit the phenomena of erythrophagocytosis.
The possibility of the transition of pernicious anemia to an aplastic state, recognized by previous authors, is currently denied. Sectional findings of red bone marrow indicate that hematopoiesis persists until the last moment of the patient’s life. The lethal outcome does not occur due to anatomical aplasia of the hematopoietic organ, but due to the fact that functionally defective megaloblastic hematopoiesis is not able to provide the vital processes of oxygen respiration for the body with the necessary minimum of red blood cells.
Etiology and pathogenesis. Since Biermer identified “pernicious” anemia as an independent disease, the attention of clinicians and pathologists has been attracted by the fact that with this disease gastric achylia is constantly observed (which, according to recent data, turned out to be histamine-resistant), and atrophy of the gastric mucosa is found in sections ( anadenia ventriculi). Naturally, there was a desire to establish a connection between the state of the digestive tract and the development of anemia.
According to modern concepts, pernicious anemic syndrome should be considered as a manifestation of endogenous B12 vitamin deficiency.
The direct mechanism of anemia in Addison-Biermer disease is that due to vitamin B12 deficiency, the metabolism of nucleoproteins is disrupted, which leads to a disorder of mitotic processes in hematopoietic cells, in particular in bone marrow erythroblasts. The slow pace of megaloblastic erythropoiesis is caused by both a slowdown in mitotic processes and a reduction in the number of mitoses themselves: instead of three mitoses characteristic of normoblastic erythropoiesis, megaloblastic erythropoiesis occurs with one mitosis. This means that while one pronormoblast produces 8 red blood cells, one promegaloblast produces only 2 red blood cells.
The disintegration of many hemoglobinized megaloblasts that did not have time to “denucleate” and turn into erythrocytes, along with their slow differentiation (“abortion of erythropoiesis”) is the main reason leading to the fact that the processes of hematopoiesis do not compensate for the processes of blood destruction and anemia develops, accompanied by an increased accumulation of unused products hemoglobin breakdown.
The latter is confirmed by data from determining the iron cycle (using radioactive isotopes), as well as increased excretion of blood pigments - urobilin, etc.
In connection with the indisputably established “deficient” endogenous vitamin deficiency nature of pernicious anemia, the previously dominant views on the significance of increased breakdown of red blood cells in this disease have undergone a radical revision.
As is known, pernicious anemia was classified as a hemolytic anemia, and megaloblastic erythropoiesis was considered as a response of the bone marrow to increased breakdown of red blood cells. However, the hemolytic theory has not been confirmed either in experiment, or in the clinic, or in medical practice. Not a single experimenter was able to obtain pictures of pernicious anemia when animals were poisoned with a hemolytic nucleus. Anemia of the hemolytic type, neither in experiment nor in the clinic, is accompanied by a megaloblastic reaction of the bone marrow. Finally, attempts to treat pernicious anemia by splenectomy to reduce the breakdown of red blood cells have also been unsuccessful.
Increased excretion of pigments in pernicious anemia is explained not so much by the destruction of newly formed red blood cells in the circulating blood, but by the disintegration of hemoglobin-containing megaloblasts and megalocytes even before their release into the peripheral blood, i.e. in the bone marrow and foci of extramedullary hematopoiesis. This assumption is confirmed by the fact that we discovered increased erythrophagocytosis in the bone marrow of patients with pernicious anemia. The increased iron content in the blood serum noted during the period of relapse of pernicious anemia is mainly explained by impaired iron utilization, since during the period of remission the blood iron content returns to normal levels.

In addition to the increased deposition in the tissues of the iron-containing pigment - hemosiderin and the increased content of iron-free pigments (bilirubin, urobilin) in the blood, duodenal juice, urine and feces, in patients with pernicious anemia an increased amount of porphyrin and small amounts of hematin are found in the blood serum, urine and bone marrow. Porphyrinemia and hematinemia are explained by insufficient utilization of blood pigments by the hematopoietic organs, as a result of which these pigments circulate in the blood and are excreted from the body in the urine.
Megaloblasts (megalocytes) in pernicious anemia, as well as embryonic megaloblasts (megalocytes), are extremely rich in porphyrin and cannot be full oxygen carriers to the same extent as normal red blood cells. This conclusion is consistent with the established fact of increased oxygen consumption by megaloblastic bone marrow.
The B12-avitaminosis theory of the genesis of pernicious anemia, generally accepted by modern hematology and clinics, does not exclude the role of additional factors contributing to the development of anemia, in particular the qualitative inferiority of macromegalocytes and their “fragments” - poikilocytes, schizocytes and the “fragility” of their presence in the peripheral blood. According to the observations of a number of authors, 50% of red blood cells transfused from a patient with pernicious anemia to a healthy recipient remain in the latter’s blood for 10-12 to 18-30 days. The maximum lifespan of erythrocytes during the period of exacerbation of pernicious anemia ranges from 27 to 75 days, therefore, 2-4 times less than normal. Finally, the weakly expressed hemolytic properties of the plasma of patients with pernicious anemia are of some (by no means primary) importance, proven by observations of erythrocytes from healthy donors transfused to patients with pernicious anemia and subjected to accelerated decay in the blood of recipients (Hamilton and co-workers, Yu. M. Bala).
The pathogenesis of funicular myelosis, as well as pernicious anemic syndrome, is associated with atrophic changes in the gastric mucosa, leading to a deficiency of the vitamin B complex.
Clinical observations that have established the beneficial effect of using vitamin B12 in the treatment of funicular myelosis allow us to recognize the nervous syndrome in Biermer's disease (along with anemic syndrome) as a manifestation of vitamin B12 deficiency in the body.
The question of the etiology of Addison-Birmer disease should still be considered unresolved.
According to modern views, Addison-Biermer disease is a disease characterized by congenital inferiority of the glandular apparatus of the fundus of the stomach, which is revealed with age in the form of premature involution of glands producing gastromucoprotein, necessary for the assimilation of vitamin B12.
We are not talking about atrophic gastritis (gastritis atrophicans), but about gastric atrophy (atrophia gastrica). The morphological substrate of this peculiar dystrophic process is nested, rarely diffuse atrophy, affecting mainly the fundic glands of the fundus of the stomach (anadenia ventriculi). These changes, which create “pearl spots” known to pathologists of the last century, are detected intravitally during gastroscopic examination (see above) or by biopsy of the gastric mucosa.
The concept of the autoimmune genesis of gastric atrophy in pernicious anemia, put forward by a number of authors (Taylor, 1959; Roitt and co-workers, 1964), is worthy of attention. This concept is supported by the detection in the blood serum of most patients with pernicious anemia of specific antibodies against the parietal and chief cells of the gastric glands that temporarily disappear under the influence of corticosteroids, as well as immunofluorescence data showing the presence of antibodies fixed in the cytoplasm of the parietal cells.
It is believed that autoantibodies against gastric cells play a pathogenetic role in the development of atrophy of the gastric mucosa and subsequent disorders of its secretory function.
By microscopic examination of the biopsied gastric mucosa, significant lymphoid infiltration was discovered in the latter, which is considered as evidence of the participation of immunocompetent cells in unleashing an organ-specific autoimmune inflammatory process with subsequent atrophy of the gastric mucosa.
In this regard, the frequency of combinations of the histological picture of atrophy and lymphoid infiltration of the gastric mucosa, characteristic of Birmer's pernicious anemia, with Hashimoto's lymphoid thyroiditis, is noteworthy. Moreover, deceased patients with Birmer's anemia often show (at autopsy) signs of thyroiditis.
The immunological commonality of Biermer's anemia and Hashimoto's thyroiditis is supported by the fact that antithyroid antibodies were detected in the blood of patients with Biermer's anemia, and, on the other hand, antibodies against parietal cells of the gastric mucosa in patients with thyroid disease. According to Irvine et al (1965), antibodies against gastric parietal cells are found in 25% of patients with Hashimoto's thyroiditis (antithyroid antibodies in these same patients are found in 70% of cases).
The results of studies of relatives of patients with Birmer's anemia are also of interest: according to various authors, antibodies against the lining cells of the gastric mucosa and against the cells of the thyroid gland, as well as a violation of the secretory and adsorption (in relation to vitamin B 12) functions of the stomach, are observed in no less than 20 % of relatives of patients with Birmer's pernicious anemia.
According to the latest studies conducted using the radiodiffusion method on 19 patients with pernicious anemia, a group of American researchers established the existence in the blood serum of all patients of antibodies that either “block” the intrinsic factor or bind both the intrinsic factor (IF) and the CF+ complex AT 12.
Anti-HF antibodies have also been found in the gastric juice and saliva of patients with Birmer's anemia.
Antibodies are also found in the blood of infants (up to 3 weeks of age) born from mothers with pernicious anemia who contained anti-HF antibodies in their blood.
In childhood forms of B12-deficiency anemia, occurring with intact gastric mucosa, but with impaired production of internal factor (see below), antibodies to the latter (anti-HF antibodies) are detected in approximately 40% of cases.
Antibodies are not detected in childhood pernicious anemia, which occurs due to impaired absorption of vitamin B 12 at the intestinal level.
In light of the above data, the deep pathogenesis of B12 deficiency Biermer's anemia appears to be an autoimmune conflict.
Schematically, the occurrence of neuroanemic (B12-deficiency) syndrome in Addison-Biermer disease can be represented as follows.

The question of the relationship between pernicious anemia and gastric cancer requires special consideration. This question has long attracted the attention of researchers. Since the first descriptions of malignant anemia, it has been known that this disease is often combined with malignant neoplasms of the stomach.
According to US statistics (cited by Wintrobe), stomach cancer occurs in 12.3% (in 36 cases out of 293) of those who died from malignant anemia over the age of 45 years. According to summary data collected by A.V. Melnikov and N.S. Timofeev, the incidence of stomach cancer in patients with malignant anemia, established on the basis of clinical, radiological and sectional materials, is 2.5%, i.e. approximately 8 times more than in the general population (0.3%). The incidence of stomach cancer in patients with pernicious anemia, according to the same authors, is 2-4 times higher than that of stomach cancer in people of the same age who do not suffer from anemia.
Noteworthy is the increase in cases of stomach cancer in patients with pernicious anemia in recent years, which should be explained by the prolongation of life of patients (due to effective Bia therapy) and the progressive restructuring of the gastric mucosa. In most cases, these are patients with pernicious anemia who develop stomach cancer. One should not, however, lose sight of the possibility that gastric cancer itself sometimes gives a picture of pernicious anemia. At the same time, it is not necessary, as some authors suggested, that the cancer should strike the fundic part of the stomach, although the localization of the tumor in this part is certainly of “aggravating” significance. According to S. A. Reinberg, out of 20 patients with a combination of stomach cancer and pernicious anemia, only 4 had the tumor localized in the cardial and subcardial regions; in 5, a tumor was found in the antrum, in 11 - in the body of the stomach. A pernicious anemic blood picture can develop at any location of gastric cancer, accompanied by diffuse atrophy of the mucosa involving the glands of the fundus of the stomach. There are cases when the developed pernicious anemic blood picture was the only symptom of stomach cancer (a similar case was described by us) 1 .
Signs suspicious of the development of a stomach cancer in a patient with pernicious anemia should be considered, firstly, a change in the type of anemia from hyperchromic to normohypochromic, secondly, the patient’s developing refractoriness to vitamin B12 therapy, thirdly, the appearance of new symptoms, uncharacteristic for pernicious anemia as such: loss of appetite, weight loss. The appearance of these symptoms obliges the doctor to immediately examine the patient in the direction of possible gastric blastoma.
It should be emphasized that even a negative result of an X-ray examination of the stomach cannot guarantee the absence of a tumor.
Therefore, in the presence of even just clinical and hematological symptoms that inspire reasonable suspicion of the development of blastoma, it is necessary to consider surgical intervention - a trial laparotomy - as indicated.
Forecast. Liver therapy, proposed in 1926, and modern treatment with vitamin B i2 radically changed the course of the disease, which had lost its “malignancy”. Now the lethal outcome of malignant anemia, which occurs during oxygen starvation of the body (anoxia) in a coma, is very rare. Although not all symptoms of the disease disappear during remission, nevertheless, persistent blood remission, which occurs as a result of systematic use of antianemic drugs, is actually tantamount to practical recovery. There are cases of complete and final recovery, especially for those patients who have not yet developed a nervous syndrome.
Treatment. For the first time, Minot and Murphy (1926) reported the cure of 45 patients with malignant anemia using a special diet rich in raw calf liver. The most active was low-fat calf liver, minced twice and prescribed to the patient 200 g per day 2 hours before meals.
A great achievement in the treatment of pernicious anemia has been the production of effective liver extracts. Of the parenterally administered liver extracts, the most famous was the Soviet campolon, extracted from the liver of cattle and produced in 2 ml ampoules. In connection with reports of the antianemic role of cobalt, liver concentrates enriched with cobalt were created. A similar Soviet drug, antianemin, was successfully used in domestic clinics to treat patients with pernicious anemia. The dosage of antianemin is from 2 to 4 ml into the muscle daily until hematological remission is obtained. Practice has shown that a single administration of a massive dose of Campolon in 12-20 ml (the so-called “Campolone blow”) is equivalent in effect to a full course of injections of the same drug, 2 ml daily.
According to modern research, the specificity of the action of liver drugs in pernicious anemia is due to the content of hematopoietic vitamin (B12) in them. Therefore, the basis for the standardization of antianemic drugs is the quantitative content of vitamin B12 in micrograms or gammas per 1 ml. Campolon of various series contains from 1.3 to 6 μg/ml, antianemin - 0.6 μg/ml of vitamin B12.
In connection with the production of synthetic folic acid, the latter was used to treat pernicious anemia. Prescribed per os or parenterally in a dose of 30-60 mg or more (maximum up to 120-150 mg pro die), folic acid causes a rapid onset of remission in a patient with pernicious anemia. However, the negative property of folic acid is that it leads to increased consumption of tissue vitamin B12. According to some data, folic acid does not prevent the development of funicular myelosis, and with long-term use even promotes it. Therefore, folic acid has not been used for Addison-Biermer anemia.
Currently, due to the introduction of vitamin B12 into widespread practice, the above remedies in the treatment of pernicious anemia, which were used for 25 years (1925-1950), have lost their significance.
The best pathogenetic effect in the treatment of pernicious anemia is achieved from parenteral (intramuscular, subcutaneous) use of vitamin B12. A distinction should be made between saturation therapy, or “impact therapy”, carried out during an exacerbation, and “maintenance therapy”, carried out during a period of remission.
Saturation therapy. Initially, based on a person’s daily need for vitamin B12, which was determined to be 2-3 mcg, it was proposed to administer relatively small doses of vitamin B12 - 15 daily or 30 every 1-2 days. At the same time, it was believed that the introduction of large doses was inappropriate due to the fact that most of the vitamin B12 obtained in excess of 30 is excreted from the body in the urine. Subsequent studies, however, showed that the B12-binding capacity of plasma (depending mainly on the content of -globulin) and the degree of utilization of vitamin B12 vary depending on the body's need for vitamin B12, in other words, on the degree of vitamin B12 deficiency in tissues . The normal content of vitamin B12 in the latter, according to Ungley, is 1000-2000 (0.1-0.2 g), of which half is in the liver.
According to Mollin and Ross, with severe B12 deficiency in the body, manifested clinically by the picture of funicular myelosis, after an injection of 1000 vitamin B12, 200-300 is retained in the body .
Clinical experience has shown that although small doses of vitamin B12 practically lead to clinical improvement and restoration of normal (or near normal) blood counts, they are still insufficient to restore tissue reserves of vitamin B12. Undersaturation of the body with vitamin B12 is manifested both in the known inferiority of clinical and hematological remission (preservation of residual phenomena of glossitis and especially neurological phenomena, macrocytosis of erythrocytes), and in a tendency to early relapses of the disease. For the reasons stated above, the use of small doses of vitamin B12 is considered inappropriate. In order to eliminate vitamin B12 deficiency during the period of exacerbation of pernicious anemia, it is currently proposed to use medium - 100-200 and large - 500-1000 - doses of vitamin B12.
Practically, as a regimen for exacerbation of pernicious anemia, we can recommend injections of vitamin B12 100-200 daily during the first week (before the onset of reticulocyte crisis) and then every other day until the onset of hematological remission. On average, with a course of treatment lasting 3-4 weeks, the course dose of vitamin B12 is 1500-3000 .
For funicular myelosis, more massive (shock) doses of vitamin B12 are indicated - 500-1000 daily or every other day for 10 days, and then 1-2 times a week until a lasting therapeutic effect is obtained - the disappearance of all neurological symptoms.
Positive results - a pronounced improvement in 11 out of 12 patients with funicular myelosis (and in 8 patients with restoration of ability to work) - were obtained by L. I. Yavorkovsky with endolubic administration of vitamin B12 at a dose of 15-200 mcg With at intervals of 4-10 days, a total of up to 840 mcg per course of treatment . Considering the possibility of complications, including severe meningeal syndrome (headache, nausea, stiff neck, fever), the indication for endolubic administration of vitamin B12 should be limited to exclusively severe cases of funicular myelosis. Other methods of treating funicular myelosis used in the recent past: spinal diathermy, raw pork stomach in large doses (300-400 g per day), vitamin B1 50-100 mg per day - have now lost their significance, with the exception of vitamin B1 , recommended for neurological disorders, especially the so-called polyneuritic form.
The duration of treatment with vitamin B12 for funicular myelosis is usually 2 months. The course dose of vitamin B12 is from 10,000 to 25,000 .
To obtain stable remission, Chevallier recommended long-term treatment with vitamin B12 in massive doses (500-1000 per day) until the highest red blood counts are obtained (hemoglobin - 100 units, red blood cells - over 5,000,000).
In connection with the long-term use of massive doses of vitamin B12, the question of the possibility of hypervitaminosis B12 arises. This issue is resolved negatively due to the rapid removal of vitamin B12 from the body. The accumulated wealth of clinical experience confirms the virtual absence of signs of oversaturation of the body with vitamin B12, even with long-term use.
Oral administration of vitamin B12 is effective in combination with the simultaneous administration of gastric antianemic factor - gastromucoprotein. Favorable results were obtained in the treatment of patients with pernicious anemia by oral administration of tablet preparations containing vitamin B12 in combination with gastromucoprotein.
In particular, positive results were noted when using the domestic drug mucovit (the drug was produced in tablets containing 0.2 g of gastromucoprotein from the mucous membrane of the pyloric part of the lower stomach and 200 or 500 mcg of vitamin B12).
In recent years, there have been reports of positive results in treating patients with pernicious anemia with vitamin B12 administered orally at a dose of at least 300 per day without intrinsic factor. In this case, you can expect that even 10% of the administered vitamin B12 will be absorbed, i.e. approximately 30 , quite sufficient to ensure the onset of hematological remission.
It is also proposed to administer vitamin B12 in other ways: sublingually and intranasally - in the form of drops or by spraying - at a dose of 100-200 mcg daily until the onset of hematological remission, followed by maintenance therapy 1-3 times a week.
According to our observations, transformation of hematopoiesis occurs within the first 24 hours after the injection of vitamin B12, and the final normalization of bone marrow hematopoiesis is completed 48-72 hours after the administration of vitamin B12.
The possibility of transforming the megaloblastic type of hematopoiesis into a normoblastic one is decided in the light of the unitary theory from the point of view of the genesis of erythroblasts of both types from a single parent cell. As a result of the onset of saturation of the bone marrow with the “erythrocyte maturation factor” (vitamin B12, folinic acid), the direction of development of basophilic erythroblasts changes. The latter, in the process of differentiating division, turn into cells of the normoblastic series.
Already 24 hours after the injection of vitamin B12, radical changes occur in hematopoiesis, expressed in the massive division of basophilic erythroblasts and megaloblasts with the differentiation of the latter into new forms of erythroblasts - mainly meso- and microgeneration. The only sign indicating the “megaloblastic past” of these cells is the disproportion between the high degree of hemoglobinization of the cytoplasm and the nucleus, which still retains its loose structure. As the cell matures, the dissociation in the development of the nucleus and cytoplasm is smoothed out. The closer a cell is to final maturation, the more it approaches a normoblast. The further development of these cells - their denuclearization, final hemoglobinization and transformation into erythrocytes - occurs according to the normoblastic type, at an accelerated pace.
On the part of granulopoiesis, there is an increased regeneration of granulocytes, especially eosinophils, among which there is a sharp shift to the left with the appearance of a significant number of eosinophilic promyelocytes and myelocytes. On the contrary, among neutrophils there is a shift to the right with an absolute predominance of mature forms. The most important is the disappearance of polysegmented neutrophils characteristic of pernicious anemia. During the same period, restoration of the normal morphophysiology of giant bone marrow cells and the normal process of platelet formation is observed.
Reticulocyte crisis occurs on the 5-6th day.
Hematological remission is determined by the following indicators: 1) the onset of a reticulocyte reaction; 2) normalization of bone marrow hematopoiesis; 3) normalization of peripheral blood; 4) restoration of normal levels of vitamin B12 in the blood.
The reticulocyte response, expressed graphically as a curve, in turn depends on the degree of anemia (it is inversely proportional to the initial number of red blood cells) and the speed of the bone marrow response. The faster the curve rises, the slower its decline, which is sometimes interrupted by a second rise (especially with irregular treatment).
Isaacs and Friedeman proposed a formula by which in each individual case one can calculate the maximum percentage of reticulocytes expected under the influence of treatment:

Where R - expected maximum percentage of reticulocytes; En - original number of red blood cells in millions.
Example. The number of red blood cells on the day of initiation of therapy was 2,500,000.

The immediate effect of vitamin B12 therapy in the sense of replenishing the peripheral blood with newly formed red blood cells begins to be felt only from the 5-6th day after the administration of the antianemic drug. The percentage of hemoglobin increases more slowly than the number of red blood cells, so the color indicator in the remission stage usually decreases and becomes less than one (Fig. 44). In parallel with the cessation of megaloblastic erythropoiesis and the restoration of a normal blood picture, the symptoms of increased breakdown of red blood cells also decrease: the yellowness of the integument disappears, the liver and spleen are reduced to normal sizes, the amount of pigments in the blood serum, bile, urine and feces decreases.

Rice. 44. Dynamics of blood parameters under the influence of vitamin B12.
Clinical remission is expressed in the disappearance of all pathological symptoms, including anemic, dyspeptic, neurological and ocular. The exception is histamine-resistant achylia, which usually persists during remission.
Improvement in general condition: increased strength, disappearance of diarrhea, drop in temperature - usually occurs before the disappearance of anemic symptoms. Glossitis is eliminated somewhat more slowly. In rare cases, restoration of gastric secretion is also noted. Nervous phenomena are reduced to some extent: paresthesia and even ataxia disappear, deep sensitivity is restored, and the mental state improves. In severe forms, nervous phenomena are hardly reversible, which is associated with degenerative changes in nervous tissue. The effectiveness of vitamin B12 therapy has a known limit, after which the increase in blood counts stops. Due to the faster increase in the number of red blood cells compared to the increase in hemoglobin, the color indicator decreases to 0.9-0.8, and sometimes lower, anemia becomes hypochromic. It seems that vitamin B12 therapy, while promoting the maximum use of iron to build red blood cell hemoglobin, leads to the depletion of its reserves in the body. The development of hypochromic anemia in this period is also favored by reduced absorption of dietary iron due to achylia. Therefore, during this period of illness, it is advisable to switch to treatment with iron preparations - Ferrum hydrogenio reductum 3 g per day (must be washed down with hydrochloric acid) or hemostimulin. An indication for the administration of iron to patients with pernicious anemia may be a decrease in plasma iron from elevated levels (up to 200-300%) during the period of exacerbation to subnormal levels during the period of remission. An indicator of the beneficial effect of iron during this period is an increase in the utilization of radioactive iron (Fe 59) from 20-40% (before treatment) to normal (after treatment with vitamin B12).
The issue of using blood transfusions for pernicious anemia is decided in each case according to the indications. An absolute indication is pernicious coma, which poses a threat to the patient’s life due to increasing hypoxemia.
Despite the brilliant achievements in the treatment of pernicious anemia, the problem of its final cure still remains unresolved. Even in the remission stage with normal blood counts, characteristic changes in erythrocytes (aniso-poikilocytosis, single macrocytes) and a shift of neutrophils to the right can be detected. Examination of gastric juice reveals in most cases permanent achylia. Changes in the nervous system can progress even in the absence of anemia.
With the cessation of the administration of vitamin B12 (in one form or another), there is a threat of relapse of the disease. Clinical observations show that relapses of the disease usually occur within 3 to 8 months after cessation of treatment.
In rare cases, relapses of the disease occur after several years. Thus, in a 60-year-old patient we observed, a relapse occurred only 7 (!) years after the complete cessation of vitamin B12 intake.
Maintenance therapy consists of prescribing a preventive (anti-relapse) intake of vitamin B12. In this case, one should proceed from the fact that a person’s daily need for it is, according to the observations of various authors, from 3 to 5 . Based on these data, it can be recommended that in order to prevent relapse of pernicious anemia, it is recommended to administer 100 or weekly 50 vitamin B12 to the patient in the form of injections 2-3 times a month.
As maintenance therapy in a state of complete clinical and hematological remission and for the prevention of relapses, oral drugs - mucovit with or without intrinsic factor (see above) can also be recommended.
Prevention. Prevention of exacerbations of pernicious anemia comes down to the systematic administration of vitamin B12. The timing and dosage are set individually (see above).
Taking into account age characteristics (usually the elderly age of patients), as well as the existing pathomorphological substrate of the disease - atrophic gastritis, considered as a pre-cancrosis condition, it is necessary to show reasonable (not excessive!) oncological vigilance in relation to each patient with pernicious anemia. Patients with pernicious anemia are subject to clinical observation with mandatory blood monitoring and X-ray examination of the gastrointestinal tract at least once a year (more often if there is suspicion).
Pernicious anemia is one of the blood diseases with a well-studied cause. All disorders in hematopoiesis are caused by a lack of vitamin B 12, so another name for the disease is B 12 deficiency anemia.
In the 19th century, anemia was considered progressive and malignant. At that time it was severe and fatal.
Another name after the names of the clinicians who studied it is Addison-Birmer disease (in the UK - Addison, in Germany - Biermer). To the lack of vitamin B 12, they added the concomitant low acidity of gastric juice.
The role of vitamin B 12 in hematopoiesis
Vitamin B 12 “works” in the body not alone, but together with other elements. Most associated with folic acid (vitamin B 9). As a result of their joint activity, protein complexes responsible for maturation and division are formed in the nuclei of cells of all tissues.
Under the influence of the B 12 + B 9 complex, red blood cells mature in the bone marrow from the cells of the erythroid germ. In a deficient state, the production of normal red blood cells slows down, and synthesis reaches only the megalocyte stage. But these cells are not able to bind hemoglobin and carry oxygen. In addition, their lifespan is very short.
Reserves of B vitamins are stored in humans in the liver. It is believed that they are enough for an adult for a period of one to five years. The requirement for vitamin B 12 is 5 mcg per day, and B 9 - from 500 to 700 mcg. Folic acid reserves are barely enough for six months. A gradual “shortage” leads to a disruption in the production of red blood cells and the development of the disease.
Why does a deficiency of essential vitamins occur?
The reasons may be:
- nutritional (depending on the composition of food);
- caused by stomach diseases with impaired absorption of the vitamin.
To maintain vitamin B 12 levels, you need to eat meat, liver, kidneys, and chicken eggs. Folic acid is found in significant quantities in vegetables (spinach), yeast, and dairy products. A deficiency in intake is observed in vegetarians, alcoholics, starving people, and in people who have had part of their stomach removed. If it is impossible for the patient to eat on his own, nutritional mixtures are administered intravenously. They should have enough vitamin B 12.
In the stomach, the vitamin is bound and protected from breakdown by food enzymes by a special glycoprotein. With atrophy of the mucous membrane of the stomach and duodenum in elderly people or with chronic gastritis, peptic ulcer disease, the glycoprotein is not produced and vitamins are lost.
Through the intestinal mucosa, a complex of glycoprotein molecules with vitamin B 12 enters the bloodstream and is transported by transcobalamins, which are formed by macrophages and leukocytes. Therefore, during leukocytosis, the vitamin accumulates in greater quantities. The process of absorption of substances from the intestine (malabsorption) is disrupted in congenital and acquired pathologies: Crohn's disease, sprue, celiac disease, intestinal lymphoma.
Pernicious anemia occurs during pregnancy, in patients with psoriasis and special types of dermatitis. In this case, the body has an increased need for the vitamin, and anemia is the result of insufficient compensation.
Clinical manifestations
Pernicious anemia typically develops gradually.
Initial symptoms:
- general weakness;
- dizziness;
- increased fatigue;
- tachycardia;
- shortness of breath on exertion.
The pronounced clinical picture includes:
- yellowing of the skin and sclera (lighter than with hepatitis);
- pain and inflammation of the tongue (glossitis);
- dull pain or a feeling of heaviness in the left hypochondrium due to an enlargement of the spleen (rarely the liver).
The disease has a cyclical course with periods of exacerbations and remissions. With each exacerbation the symptoms become more severe.
Picture of tongue inflammation (glossitis)
Damage to the nervous system
With pernicious anemia, unlike other types of anemia, damage occurs to the myeloid sheath of the nerve tracts (funicular myelosis).
It manifests itself:
- impaired sensitivity in the arms and legs, numbness;
- pain in the limbs;
- "tingling" feeling;
- increasing muscle weakness to the point of atrophy;
- unsteady gait.
If left untreated, damage to the spinal cord and its roots occurs. In this case, the pathology spreads from the legs higher. First, a violation of deep sensitivity is recorded, then hearing and smell decrease.
In severe cases, it develops:
- exhaustion,
- loss of reflexes,
- limb paralysis,
- memory loss.
Visual and auditory hallucinations and delusions are possible.
Anemia and pregnancy
Pernicious anemia can occur in the second half of pregnancy. The general symptoms of anemia (dizziness, weakness, tachycardia, shortness of breath, pallor) are accompanied by a slight decrease in the sensitivity of the fingers and digestive disorders.
Pregnant women should have regular blood tests to detect symptoms early.
In advanced forms of B 12 deficiency anemia, the risk of miscarriage due to placental abruption, premature birth, and stillbirth is increased.
Treatment of the woman leads to a complete recovery.
Why do children get sick?
In childhood, the disease often develops in families with hereditary pathologies of the stomach or intestines. This leads to impaired absorption of vitamins. Less commonly, the reason lies in the nursing mother’s failure to comply with the diet and regimen.
Hereditary manifestations are detected from the age of three months. More complete symptoms develop by the age of three.
The child has:
- paleness with a lemon tint;
- dry, flaky skin;
- inflammation of the tongue;
- weight loss due to loss of appetite;
- frequent diarrhea.
Children with pernicious anemia are more susceptible to infections and get sick often. Possible developmental delay.
Diagnostics
The diagnosis is made by comparing clinical manifestations and blood patterns. When deciphering a blood test, the following is noted:
- decrease in the number of red blood cells;
- increased color index;
- changes in the size and shape of red blood cells;
- the presence of megaloblasts, erythrocytes with nuclear remains;
- decrease in the number of reticulocytes;
- shift of the leukocyte formula to the left;
- decrease in platelet count.
Treatment
Therapy for pernicious anemia begins with the administration of a balanced diet. To compensate for the necessary need for vitamins B 12 and B 9 in the daily diet, you need to include beef (tongue, heart), rabbit, chicken eggs, seafood, cottage cheese and dairy products, and legumes. Fatty foods will have to be limited because they slow down hematopoiesis.

Cyanocobolamine should not be mixed in the same syringe with other medications.
Treatment for diseases of the stomach and intestines is mandatory.
In order to compensate for the lack of vitamins, a large dose of Cyanocobalamin is administered intravenously. Feeling better occurs after a few days.
The course of treatment lasts up to a month or more, depending on the achievement of stable normalization of blood test results and the severity of the patient’s condition. Then the medicine is administered for another six months once a week.
Preparations from liver extracts (Campolon and Antianemin) are administered intramuscularly daily.
Folic acid tablets are prescribed orally.
Currently, the disease is classified as a rare type of anemia. This is facilitated by simple diagnosis and affordable treatment.
When there is a lack of vitamin B12 in the bone marrow, normal red blood cell precursor cells are replaced by megaloblasts - abnormally large cells that are unable to transform into red blood cells. If left untreated, the patient develops anemia and nerve degeneration.
General information
Pernicious anemia was first described by Addison in 1855, describing the disease as “idiopathic anemia” (anemia of unknown origin).
A detailed clinical and anatomical description of the disease belongs to Birmer (1868). It was Brimer who gave the disease the name “pernicious anemia”, i.e. pernicious anemia.
For a long time the disease was considered incurable, but in 1926 Minot and Murphy made a discovery that pernicious anemia can be cured with raw liver (liver therapy). This discovery and subsequent work by the American physiologist W. B. Castle formed the basis of modern ideas about the pathogenesis of this disease.
W. B. Castle established that normally a person produces not only hydrochloric acid and pepsin, but also a third (internal) factor - a complex compound consisting of peptides and mucoids, which is secreted by mukocytes (cells of the gastric mucosa). This compound forms a labile complex with an external factor (vitamin B12), which, after entering the blood plasma, forms a protein-B12-vitamin complex that accumulates in the liver. This complex takes part in hematopoiesis. W. B. Castle revealed the absence of internal factor secretion in the stomach in patients with pernicious anemia, but did not establish the chemical nature of the external factor.
The substance (vitamin B12), which plays the role of an external factor, was established in 1948 by Ricks and Smith.
The disease is quite common - the prevalence rate is 110 -180 patients per 100,000 population. Residents of the UK and the Scandinavian Peninsula are most susceptible to the disease.
In most cases, pernicious anemia affects people who belong to the older age group (observed in 1% of people over 60 years of age). If there is a family predisposition to the disease, the disease is detected at a younger age.
In women, the disease is observed more often (10:7 in ratio to males).
Forms
Pernicious anemia, depending on the amount of hemoglobin in the blood of patients, is divided into:
- mild disease, which is diagnosed when hemoglobin is from 90 to 110 g/l;
- moderate anemia, detected when hemoglobin is from 90 to 70 g/l;
- severe anemia, in which the blood contains less than 70 g/l of hemoglobin.
Depending on the cause of the development of B12 deficiency anemia, there are:
- Food or nutritional anemia (develops in young children). It is observed when there is a lack of vitamin B12 in the diet (vegetarians, premature babies and children fed with milk powder or goat milk).
- Classic B12-deficiency anemia associated with atrophy of the gastric mucosa and the absence of “intrinsic” factor.
- Juvenile B12 deficiency anemia, which develops due to the functional insufficiency of the fundic glands that produce glandular mucoprotein. The gastric mucosa and the secretion of hydrochloric acid are preserved. This disease is reversible.
Separately, there is familial B12 deficiency anemia (Olga Imerslund's disease), which is caused by impaired transport and absorption of vitamin B12 in the intestines. In patients with this disease, protein is detected in the urine (proteinuria).
Reasons for development
Pernicious anemia develops in the body:
The causes of the disease also include an autoimmune factor - in 90% of patients, the presence of circulating autoantibodies to stomach cells that secrete hydrochloric acid and intrinsic Castle factor was detected (present in 5-10% of healthy people), and in 60% of patients - antibodies to intrinsic factor Castle.
Pathogenesis
Normally, 6-9 mcg of vitamin B12 per day enters the human body with food (2-5 mcg is excreted, and about 4 mcg is retained in the body). Since the reserves of vitamin B12 in the body are significant, malignant anemia develops only after a long period (about 4 years) after its supply has ceased or its absorption has been impaired.
Insufficiency of cyanocobalamin (vitamin B12) leads to a deficiency of its coenzyme forms - methylcobalamin and 5-deoxyadenosylcobalamin. Methylcobalamin is necessary for the normal course of the formation of red blood cells, and 5-deoxyadenosylcobalamin ensures metabolic processes occurring in the central nervous system and peripheral nervous system.
With a deficiency of methylcobalamin, the synthesis of nucleic acids and essential amino acids is disrupted and the megaloblastic type of hematopoiesis develops. In the process of formation and maturation, red blood cells take the form of megaloblasts and megalocytes, which are quickly destroyed and are not able to perform an oxygen transport function. As a result, the number of red blood cells in the peripheral blood is significantly reduced and an anemic syndrome develops.
An insufficient amount of 5-deoxyadenosylcobalamin causes a disturbance in the metabolism of fatty acids, which provoke the accumulation of toxic methylmalonic and propionic acids in the body. These acids have a damaging effect on the neurons of the brain and spinal cord, contribute to disruption of myelin synthesis and degeneration of the myelin layer, so pernicious anemia is accompanied by damage to the nervous system.
Symptoms
Pernicious anemia manifests itself:
- Anemic syndrome, which is accompanied by general weakness, decreased performance, low-grade fever, dizziness, and fainting. The syndrome is also manifested by shortness of breath, which occurs even with minor exertion, and flickering. The skin becomes pale with a slightly yellowish tint, and the face becomes puffy. Auscultation of the heart may reveal systolic murmurs, and with prolonged anemia, myocardial dystrophy and heart failure develop.
- Gastroenterological syndrome, which is accompanied by nausea and vomiting, decreased appetite and body weight, and constipation. Gunter's glossitis also occurs (the structure of the tongue changes due to a lack of vitamin B12), in which the tongue acquires a crimson or bright red hue, and its surface becomes smoothed, “varnished.” The patient experiences a burning sensation in the tongue. It is possible to develop angular (localized in the corners of the mouth) stomatitis. Gastric secretion is significantly reduced; gastroscopy reveals atrophic changes in the gastric mucosa.
- Neurological syndrome. With a deficiency of vitamin B12, muscle weakness is observed, the gait becomes unstable, the legs become stiff, and the patient experiences numbness in the limbs. Long-term deficiency of vitamin B12 leads to damage to the spinal cord and brain (vibration, pain and tactile sensitivity disappears, convulsions occur). Examination reveals increased tendon reflexes, the presence of Romberg's symptom (loss of balance with eyes closed) and Babinski's reflex (extension of the first toe during streak irritation of the skin of the outer edge of the sole), signs of funicular myelosis.
Megaloblastic anemia may be accompanied by irritability, low mood, and difficulty urinating. Sometimes impotence and visual disturbances develop.
If the brain is damaged, the perception of yellow and blue colors may be impaired; in rare cases, hallucinations and other mental disorders are observed.
Diagnostics
Pernicious anemia is diagnosed using:
- Analysis of patient complaints and medical history, during which the doctor clarifies the duration of the disease, the presence of hereditary and concomitant diseases, etc.
- Physical examination findings. During the examination, the doctor pays attention to the shade of the skin, pulse and blood pressure (with B12-deficiency anemia, the pulse is often rapid and blood pressure is reduced). The tongue must be examined.
- Laboratory test data.
Laboratory tests include:
- A blood test that reveals a decrease in the number of red blood cells, an increase in their size, a decrease in red blood cell precursor cells (reticulocytes), a decreased level of hemoglobin, a decreased content of platelets and an increase in their size. A change in the color indicator (the ratio of the first three digits of the number of red blood cells and a 3-fold increase in hemoglobin level) is also detected - in the direction of increase - with a norm of 0.86 to 1.05, with pernicious anemia this ratio exceeds 1.05.
- Urinalysis, which allows you to identify concomitant diseases (pyelonephritis, etc.), as well as suggest a hereditary form of the disease.
- A biochemical blood test to detect a decrease in the level of vitamin B12 in the blood, determine the level of cholesterol, uric acid, glucose, and detect creatinine (a protein breakdown product). Megaloblastic anemia is often accompanied by an increase in the level of bilirubin, formed from the breakdown of red blood cells, in the level of iron as a result of a decrease in its use in the formation of new red blood cells, and in the level of the enzyme lactate dehydrogenase, which accelerates chemical reactions.
To examine the bone marrow under general anesthesia, a puncture is made in the area of the anterior and posterior iliac spines (to avoid data distortion, the study is carried out before vitamin B12 is prescribed). Analysis of the myelogram allows us to detect the megaloblastic type of hematopoiesis and increased formation of red blood cells.
In addition, they carry out:
- ECG, which can detect an increased heart rate and, in some cases, irregular heart rhythms.
- Gastroscopy, which reveals the absence of hydrochloric acid in gastric juice (achlorhydria) and atrophic gastritis, affecting the sections in which the secretion of hydrochloric acid and pepsin occurs. Since gastric epithelial cells look atypical during cytological examination, a differential diagnosis is made with gastric cancer.
- X-ray of the stomach, ultrasound of the abdominal organs.
- MRI of the brain and examination by a neurologist.
Vitamin B12 absorption is assessed using the Schilling test. The patient takes radioactive vitamin B12 orally, and a few hours later he is given a “loading” dose of the unlabeled vitamin parenterally. The content of radioactive vitamin in 24-hour urine is then measured. With preserved kidney function, a decrease in its secretion indicates a decrease in the absorption of vitamin B12 in the intestine.
Treatment
Treatment of megaloblastic anemia is aimed at eliminating the cause of the disease and normalizing hematopoiesis. Therapy includes:
- Treatment of gastrointestinal diseases (for autoimmune damage to the stomach, glucocorticosteroids are prescribed), a balanced diet, which includes fermented milk products, beef, seafood, eggs and rabbit meat.
- The use of praziquantel or phenasal for diphyllobothriasis.
- Replenishment of vitamin B12 deficiency.
Pernicious anemia is treated by administering cyanocobalamin (vitamin B12) for 4 to 6 weeks, 200 to 500 mg subcutaneously once a day. Then the vitamin is administered once a week (a course of 3 months), and then they move on to injections 2 times a month for six months (the dose does not change).
Normalization of hematopoiesis occurs approximately 2 months after the start of treatment (the exact timing depends on the severity of the anemia).
Red blood cell transfusion is performed only in cases of anemic coma or severe anemia.
Prevention
Prevention of the disease comes down to:
- nutritious nutrition;
- timely treatment of diseases causing vitamin B12 deficiency;
- taking a maintenance dose of cyanocobalamin after removal of part of the stomach or intestines.